Review session Bonding Name
advertisement
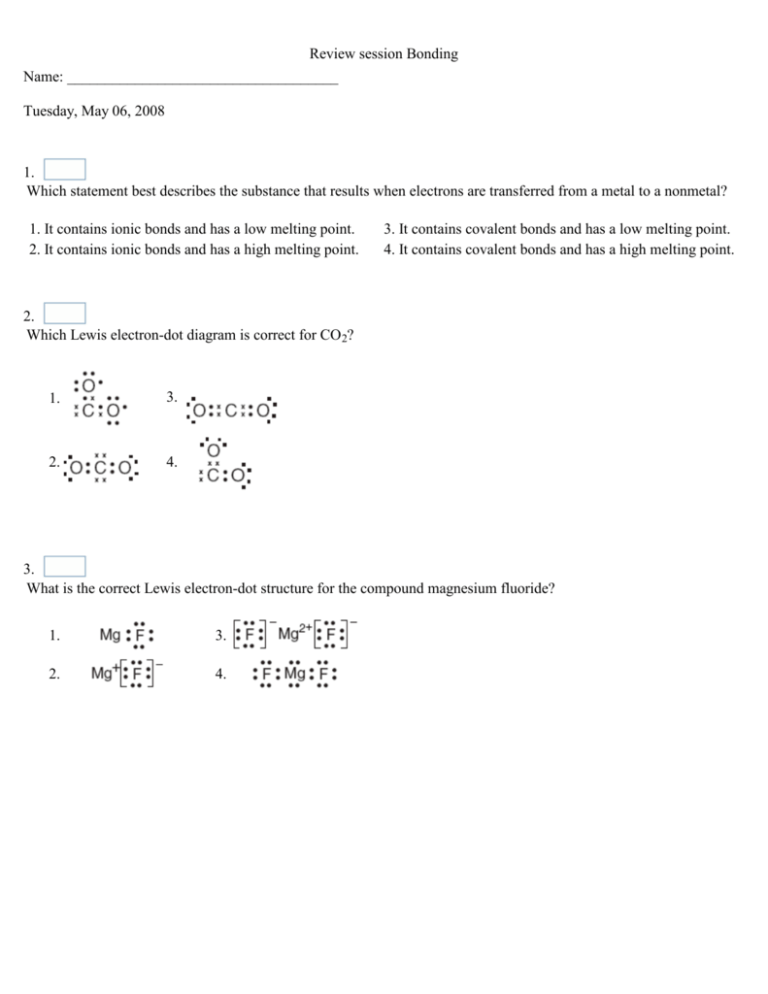
Review session Bonding Name: ____________________________________ Tuesday, May 06, 2008 1. Which statement best describes the substance that results when electrons are transferred from a metal to a nonmetal? 1. It contains ionic bonds and has a low melting point. 2. It contains ionic bonds and has a high melting point. 3. It contains covalent bonds and has a low melting point. 4. It contains covalent bonds and has a high melting point. 2. Which Lewis electron-dot diagram is correct for CO 2? 1. 3. 2. 4. 3. What is the correct Lewis electron-dot structure for the compound magnesium fluoride? 1. 3. 2. 4. Review session Bonding 4. Which electron-dot diagram best represents a compound that contains both ionic and covalent bonds? 1. 3. 2. 4. 5. The electrons in a bond between two iodine atoms (I2) are shared 1. equally, and the resulting bond is polar 2. equally, and the resulting bond is nonpolar 3. unequally, and the resulting bond is polar 4. unequally, and the resulting bond is nonpolar 6. Which elements combine by forming an ionic bond? 1. sodium and potassium 2. sodium and oxygen 3. carbon and oxygen 4. carbon and sulfur 7. Which substance is correctly paired with its type of bonding? 1. NaBr - nonpolar covalent 2. HCl - nonpolar covalent 3. NH3 - polar covalent 4. Br2 - polar covalent 8. Which formula represents a nonpolar molecule? 1. H2S 2. HCl 3. CH4 4. NH3 Review session Bonding 9. Which bond is most polar? 1. H-F 2. H-Cl 3. H-Br 4. H-I 10. Based on bond type, which compound has the highest melting point? 1. CH3OH 3. CaCl2 2. C6H14 4. CCl4 11. Which formula represents a nonpolar molecule? 1. HCl 2. H2O 3. NH3 4. CF4 12. Which structural formula represents a linear nonpolar molecule containing two polar bonds? 1. 3. 2. 4. Review session Bonding 13. Given the formula of a substance: What is the total number of shared electrons in a molecule of this substance? 1. 22 2. 11 3. 9 4. 6 14. Figure 1 The table lists the melting points of various substances. Based on this table, which type of substance has the highest melting point? 1. nonpolar covalent 2. polar covalent 3. ionic 4. metallic 15. Given the balanced equation: I + I I2 Which statement describes the process represented by this equation? 1. A bond is formed as energy is absorbed. 3. A bond is broken as energy is absorbed. 2. A bond is formed and energy is released. 4. A bond is broken and energy is released. Review session Bonding 16. Which molecule contains a polar covalent bond? 1. 3. 2. 4. 17. Which type of molecule is CF 4? 1. polar, with a symmetrical distribution of charge 2. polar, with an asymmetrical distribution of charge 3. nonpolar, with a symmetrical distribution of charge 4. nonpolar, with an asymmetrical distribution of charge 18. Two atoms with an electronegativity difference of 0.4 form a bond that is 1. ionic, because electrons are shared 2. ionic, because electrons are transferred 3. covalent, because electrons are shared 4. covalent, because electrons are transferred 19. Which electron-dot structure represents a non-polar molecule? 1. 3. 2. 4. Review session Bonding 20. Which diagram below shows the correct Lewis electron-dot diagram for a molecule of phosphorus trichloride, PCl 3? 1. 3. 2. 4. 21. A chemical bond between two atoms results from a simultaneous 1. attraction by the protons for the neutrons 2. attraction by the two nuclei for the electrons 3. repulsion by the valence electrons of the atoms 4. repulsion by the protons in the two nuclei 22. Which molecule contains a nonpolar covalent bond? 1. 3. 2. 4. 23. Which compound contains ionic bonds? 1. NO 2. NO2 3. CaO 4. CO2 Review session Bonding 24. Which diagram best represents a polar molecule? 1. 3. 2. 4. 25. Given the reaction system in a closed container at equilibrium and at a temperature of 298 K: The measurable quantities of the gases at equilibrium must be 1. decreasing 2. increasing 3. equal 4. constant 26. In which compound have electrons been transferred to the oxygen atom? 1. CO2 2. NO2 3. N2O 4. Na2O 27. A substance that has a melting point of 1074 K conducts electricity when dissolved in water, but does not conduct electricity in the solid phase. The substance is most likely 1. an ionic solid 2. a network solid 3. a metallic solid 4. a molecular solid Review session Bonding 28. Figure 2 The table lists four different chemical bonds and the amount of energy released when 1 mole of each of the bonds is formed. Which bond is the most stable? 1. H-F 2. H-Cl 3. H-Br 4. H-I 29. Which statement is true concerning the reaction N(g) + N(g) N2(g) + energy? 1. A bond is broken and energy is absorbed. 2. A bond is broken and energy is released. 3. A bond is formed and energy is absorbed. 4. A bond is formed and energy is released. 30. Which formula represents a nonpolar molecule containing polar covalent bonds? 1. H2O 3. NH3 2. CCl4 4. H2 31. The bonds between hydrogen and oxygen in a water molecule are classified as 1. polar covalent 2. nonpolar covalent 3. ionic 4. metallic Review session Bonding 32. Which electron-dot formula represents a polar molecule? 1. 3. 2. 4. 33. Which formula represents a molecular substance? 1. CaO 2. CO 3. Li2O 4. Al2O3 34. What is the total number of electrons shared in a double covalent bond between two atoms? 1. 1 2. 2 3. 8 4. 4 35. A substance was found to be a soft, nonconducting solid at room temperature. The substance is most likely 1. a molecular solid 2. a network solid 3. a metallic solid 4. an ionic solid Review session Bonding 36. A chemist performs the same tests on two homogeneous white crystalline solids, A and B. The results are shown in the table below. Solid A Melting Point High, 801°C Solubility in H2O 35.7 (grams per 100.0 g H2O at 0°C) Electrical Conductivity (in aqueous solution) Solid B Low, decomposes at 186°C 3.2 Good conductor Nonconductor The results of these tests suggest that 1. both solids contain only ionic bonds 2. both solids contain only covalent bonds 3. solid A contains only covalent bonds and solid B contains only ionic bonds 4. solid A contains only ionic bonds and solid B contains only covalent bonds 37. Which of the following elements has the highest electronegativity? 1. H 2. K 3. Al 4. Ca 38. When metals combine with nonmetals, the metallic atoms tend to 1. lose electrons and become positive ions 2. lose electrons and become negative ions 3. gain electrons and become positive ions 4. gain electrons and become negative ions 39. When combining with nonmetallic atoms, metallic atoms generally will 1. lose electrons and form negative ions 2. lose electrons and form positive ions 3. gain electrons and form negative ions 4. gain electrons and form positive ions Review session Bonding 40. Which factor distinguishes a metallic bond from an ionic bond or a covalent bond? 1. the mobility of electrons 2. the mobility of protons 3. the equal sharing of electrons 4. the unequal sharing of electrons 41. Metallic bonding occurs between atoms of 1. sulfur 2. copper 3. fluorine 4. carbon 42. Based on electronegativity values, which type of elements tends to have the greatest attraction for electrons in a bond? 1. metals 3. nonmetals 2. metalloids 4. noble gases 43. Which is the correct electron-dot formula for a molecule of chlorine? 1. 2. 3. 4. 44. A white crystalline salt conducts electricity when it is melted and when it is dissolved in water. Which type of bond does this salt contain? 1. ionic 2. metallic 3. covalent 4. network Review session Bonding 45. Which formula represents an ionic compound? 1. H2 2. CH4 3. CH3OH 4. NH4Cl 46. Which structural formula represents a dipole? 1. 3. 2. 4. 47. Which formula represents an ionic compound? 1. NaCl 2. N2O 3. HCl 4. H2O 48. When a chemical bond is broken, energy is 1. absorbed, only 2. released, only 3. both absorbed and released 4. neither absorbed nor released Review session Bonding 49. When a metal atom combines with a nonmetal atom, the nonmetal atom will 1. lose electrons and decrease in size 2. lose electrons and increase in size 3. gain electrons and decrease in size 4. gain electrons and increase in size 50. The degree of polarity of a chemical bond in a molecule of a compound can be predicted by determining the difference in the 1. melting points of the elements in the 3. electronegativities of the bonded atoms in a molecule of the compound compound 2. densities of the elements in the compound 4. atomic masses of the bonded atoms in a molecule of the compound Review session Bonding Answer Key for Review session Bonding 1. 2 2. 3 3. 3 4. 2 5. 2 6. 2 7. 3 8. 3 9. 1 10. 3 11. 4 12. 2 13. 1 14. 4 15. 2 16. 3 17. 3 18. 3 19. 2 20. 3 21. 2 22. 3 23. 3 24. 3 25. 4 26. 4 27. 1 28. 1 29. 4 30. 2 31. 1 32. 3 33. 2 34. 4 35. 1 36. 4 37. 1 38. 1 39. 2 40. 1 41. 2 42. 3 43. 4 44. 1 45. 4 46. 1 Review session Bonding 47. 1 48. 1 49. 4 50. 3







