Study Packet Part 2
advertisement
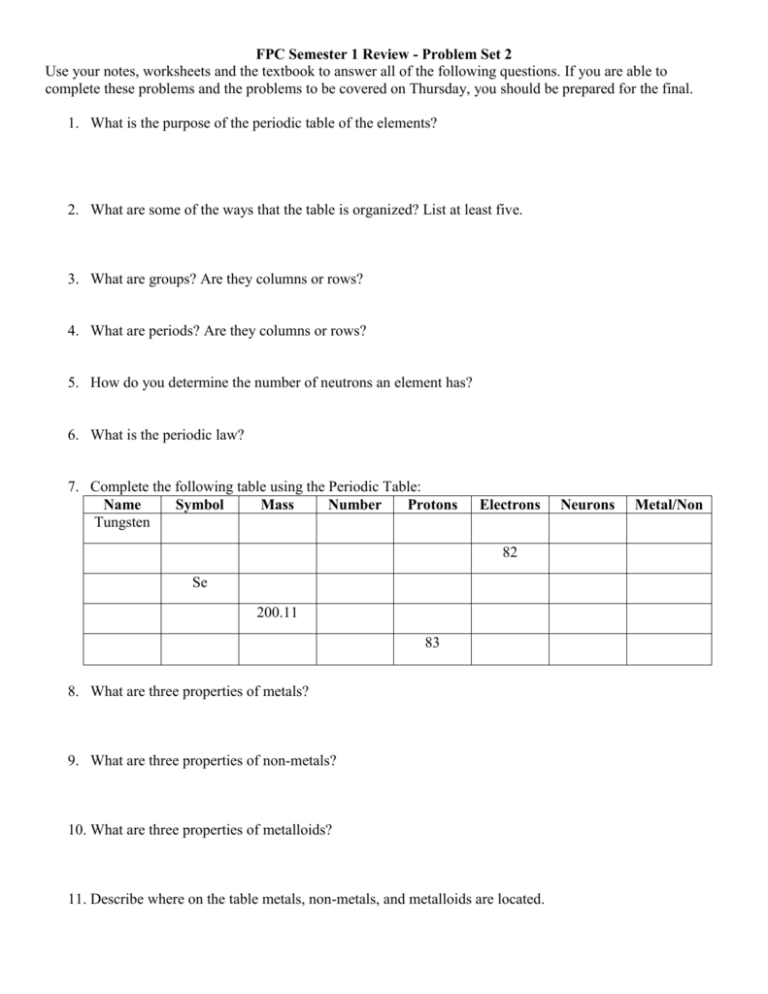
FPC Semester 1 Review - Problem Set 2 Use your notes, worksheets and the textbook to answer all of the following questions. If you are able to complete these problems and the problems to be covered on Thursday, you should be prepared for the final. 1. What is the purpose of the periodic table of the elements? 2. What are some of the ways that the table is organized? List at least five. 3. What are groups? Are they columns or rows? 4. What are periods? Are they columns or rows? 5. How do you determine the number of neutrons an element has? 6. What is the periodic law? 7. Complete the following table using the Periodic Table: Name Symbol Mass Number Protons Tungsten Electrons 82 Se 200.11 83 8. What are three properties of metals? 9. What are three properties of non-metals? 10. What are three properties of metalloids? 11. Describe where on the table metals, non-metals, and metalloids are located. Neurons Metal/Non 12. What are valence electrons? 13. How can one determine the number of valence electrons an element has by looking at the Periodic Table? 14. What is a Lewis Dot structure? What is the purpose of this structure? 15. Draw a Lewis Dot structure for the following elements: a. Calcium b. Antimony c. Xenon d. Potassium e. Oxygen 16. What is the octet rule? 17. How does the octet rule relate to Lewis Dot structures and chemical bonding? 18. What is a chemical bond? 19. What types of elements are involved in a covalent bond? 20. How do electrons interact in a covalent bond? 21. What types of elements are involved in an ionic bond? 22. How do electrons interact in an ionic bond? 23. Determine the compound suffixes for each of the following molecules given their charge: a. Mg2+ and Cl1b. Na1+ and F1c. H1+ and O224. Determine if the following molecules are ionic or covalent: a. Magnesium and Chlorine b. Beryllium and Fluorine c. Hydrogen and Oxygen d. Iron and Sulfate e. Nitrogen and Nitrogen 25. What is the rule for naming ionic molecules? 26. What is the rule for naming covalent molecules? 27. Name the following compounds using naming rules: a. P2O5 b. FeSO4 c. Mg2F d. NaCl 28. What are the five types of chemical reactions? 29. What are the general rules for each of the five types of chemical reactions? 30. Classify the following types of chemical reactions: a. b. c. d. e. ______________ Fe + CuSO4 FeSO4 + Cu ______________ 2P + 3Cl2 2PCl3 ______________ HCl + AgNO3 HNO3 + AgCl ______________ C7H16 + 11O2 7CO2 + 8H2O ______________ 2NaCl 2Na + Cl2 31. What is the conservation of mass? 32. How does the conservation of mass relate to balancing chemical reactions? 33. Why do chemical reactions need to be balanced? 34. Balance the following equations: a. ____ AgNO3 + ____ Cu ____ Cu(NO3)2 + ____ Ag b. ____ CF4 + ____ Br2 ____ CBr4 + ____ F2 c. ____ HCN + ____ CuSO4 ____ H2SO4 + ____ Cu(CN)2 d. ____ GaF3 + ____ Cs ____ CsF + ____ Ga e. ____ BaS + ____ PtF2 ____ BaF2 + ____ PtS 35. What is the pH scale? 36. What are the properties of acids? Where do they fall on the pH scale? 37. What are the properties of bases? Where do they fall on the pH scale? 38. What is the difference between a weak acid/base and a strong acid/base? 39. Explain neutralization as it relates to acids and bases.











