Chemistry Bonding Review Sheet

Review Sheet – Chapter 7 & 8 Test – Bonding
1.
Define valence electrons.
2.
Be able to determine valence electrons of all main group elements.
3.
What is the octet rule?
4.
What do atoms do obey the octet rule?
5.
When an atom does obey the octet rule, what elements is it imitating?
6.
Be able to determine the charge on atoms that either gain or lose electrons.
7.
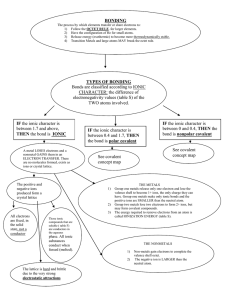
What is an ionic bond?
8.
How is an ionic compound represented? Define it.
9.
How does that affect the properties of ionic compounds? What are those properties?
10.
What types of elements generally form ionic compounds?
11.
What is a metallic bond?
12.
How does that affect the properties of metals?
13.
What is a covalent compound?
14.
How is a molecule represented? Define it.
15.
How do covalent compounds obey the octet rule?
16.
What are the three types of covalent bonds? Define them.
17.
What are the two methods for showing bonding in a molecule?
18.
What is the range of electronegativity differences for an ionic bond? Polar covalent? Nonpolar covalent?
19.
What is the difference between a polar and a nonpolar covalent bond?
20.
How do we represent polarity of a bond?
21.
Name and draw the five shapes of molecules.
22.
What is used to determine molecular shape?
23.
What is the maximum angle between bonds?
24.
How does the increased number of unshaired pairs of electrons affect the tetrahedral bond angle?
25.
How are equal bond angles explained in atoms that have electrons in the s and p orbitals? Give an example.
26.
What is hydrogen bonding?
27.
What are dipole interactions?
28.
What are dispersion forces?
29.
What is the weakest of all intermolecular forces?
30.
Draw the dot structure and the structural formula with correct shape and polarity for: CS
2
, CH
4
, BCl
3
, NH
3
, N
2
, H
2
S