Important Word Roots
advertisement

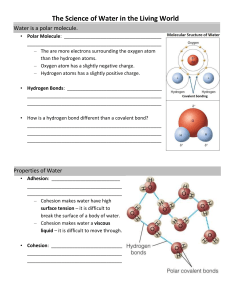
Bio 3 B Lab#2 -­‐ Water www.physicalgeography.net www.phys icalgeography.net Lab#2: Properties of water WATER LAB Important Word Roots Water • Old English w æterian "moisten” www.ces.fau.edu www.personal.psu.edu Polar • Latin polus "an end of an axis +/-­‐ Hydrogen Bonding • Hydro = w ater • Joined • *gen = a combining form meaning “that which produces” When in doubt – Say Hydrogen Bonding! www.personal.psu.edu Proff. Zannie Dallara 1 Bio 3 B Lab#2 -­‐ Water Polar, Non Polar and Amphipathic • Polar • Non-­‐Polar • Amphipathic • Combining Form • Experience www.york.ac.uk www.marin.edu Important Word Roots Adhesion Cohesion Capillary Action • French adhésion = • French cohésion • Capillaris "of hair” = sticking sticking t o • Action = Move together Add to another Water sticking to substance water http://water.usgs.gov/edu/adhesion.html springtolife.weebly.com Important Word Roots Hydro • Water *Phylic • Loving *Phobic • Fearing A charge is what makes something hydrophilic Proff. Zannie Dallara 2 Bio 3 B Lab#2 -­‐ Water WATER AND LIFE – Life on Earth began in w ater and evolved t here for 3 billion years. • Modern life remains tied to water. • Your cells are composed of 70%–95% water. – The abundance of w ater is a major reason Earth is habitable. Figure 2 .9 Water’s Life-­‐S upporting Properties • The polarity (charge) of water molecules and the hydrogen bonding that results explain most of water’s life-­‐supporting properties. 1. Water molecules s tick together. 2. Water h as a s trong resistance to change in temperature. 3. Frozen water floats. 4. Water is a common s olvent for life. www.ces.fau.edu Water’s Life-­‐S upporting Properties – The polarity ( charge) of water molecules and the hydrogen b onding that results explain most of water’s life-­‐supporting p roperties. • Water molecules stick together. – Cohesion • Water has a strong resistance to change in temperature. • Frozen water floats. • Water is a common solvent for life. Proff. Zannie Dallara 3 Bio 3 B Lab#2 -­‐ Water – Surface tension is the measure of h ow d ifficult it is to stretch or b reak the s urface of a liquid. • Hydrogen bonds give water an unusually high surface tension. Water’s Life-­‐S upporting Properties – The polarity ( charge) of water molecules and the hydrogen b onding that results explain most of water’s life-­‐supporting p roperties. • Water molecules stick together. • Water has a strong resistance to change in temperature. – Hydrogen bonds • Frozen water floats. • Water is a common solvent for life. Water’s Life-­‐S upporting Properties – The polarity ( charge) of water molecules and the hydrogen b onding that results explain most of water’s life-­‐supporting p roperties. • Water molecules stick together. • Water has a strong resistance to change in temperature. • Frozen water floats. • Water is a common solvent for life. Proff. Zannie Dallara 4 Bio 3 B Lab#2 -­‐ Water The Biological Significance of Ice Floating – When water molecules get cold enough, they move apart, forming ice. Cold = Less Energy = Less movement = More O rganized = More space = floats • • If ice did not float, ponds, lakes, and even t he oceans would freeze solid. Life in w ater could not survive if bodies of water froze solid. Water’s Life-­‐S upporting Properties – The polarity ( charge) of water molecules and the hydrogen b onding that results explain most of water’s life-­‐supporting p roperties. • Water molecules stick together. • Water has a strong resistance to change in temperature. • Frozen water floats. • Water is a common solvent for life. Water as the Solvent of Life – A solution is a liquid consisting of a homogeneous (looks the same throughout) mixture of two or more substances. • The dissolving agent is the solvent. • The dissolved substance is the solute. – When water is t he solvent, t he result is an aqueous solution. Salt crystal Sodium ion in solution Na + Na + – Cl Cl– Chloride ion in solution Proff. Zannie Dallara 5 Bio 3 B Lab#2 -­‐ Water Figure 2.14 Hydrogen bond Liquid water Hydrogen bonds constantly break and re-­form. Ice Stable hydrogen bonds hold molecules apart, making ice less dense than water. Water as the Solvent of Life – A solution is a liquid consisting of a homogeneous mixture of two or more substances. • The dissolving agent is the solvent. • The dissolved substance is the solute. – When water is the solvent, the result is an aqueous solution. © 2 0 1 3 P earso n Ed u catio n , In c. Other Uses of Water Synthesis of Polymers • Condensation Reaction (Dehydration Reaction) • Molecule of water is released – One molecule contributes –OH – Other molecule contributes –H • This is repeated as the polymer elongates www.buzzle.com hidrateh2o.com Proff. Zannie Dallara 6 Bio 3 B Lab#2 -­‐ Water Other Uses of Water Breakdown of Polymers • Hydrolysis Reaction • Break with addition of water – A –H is added t o one monomer – A –OH is added t o other monomer nerf.wikia.com www.memrise.com Other Molecules Detergent -­‐ ßHydrophilic ßHydrophobic www.york.ac.uk Other Molecules Oil ßHydrophilic ßHydrophobic wps.prenhall.com www.emec.com.eg Proff. Zannie Dallara 7 Bio 3 B Lab#2 -­‐ Water Capillary Action • Skinny tube • Touch tube to drop of water on desk • Shake out the water • Do that a couple times • Record observations • Answer questions Water ’s Life Supporting Properties The S heer Awesomeness that are Hydrogen Bonds 1. Water m olecules stick together. 2. Water has a strong resistance to change in temperature. 3. Frozen water floats. 4. Water is a common solvent for life. Proff. Zannie Dallara 8