Aspirin
advertisement

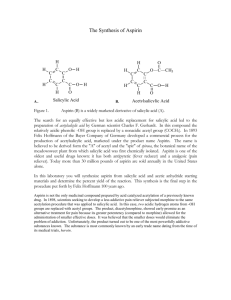
Aspirin General discussion A simple example of an organic reaction is the formation of an ester from an acid and an alcohol. The OH group on the acid and the H on the alcohol react to form water, and the carbon on the acid group bonds to the O on the alcohol group: O—H R—C H—O—R' + O—R' R—C + 1 H2O O O Generic Acid Generic Alcohol Generic Ester Water (The molecules are lined up in a way that emphasizes reaction sites) In this generic reaction, R and R' stand for H atoms or organic fragments like CH3, C2H5, or more complex aromatic groups. To follow the reaction, notice how the O‑R' on the generic alcohol ends up attached to the acid carbon on the generic ester. There are many esters, since there are many organic acids and alcohols, but they can all be formed, in principle at least, by the reaction shown above. The driving force for the reaction is generally not very great, so that one ends up with an equilibrium mixture of ester, water, acid, and alcohol. There are some esters which are solids because of their high molecular mass or other properties. Most of these esters are not soluble in water, so they may be separated from the reaction mixture by crystallization. This experiment deals with an ester of this type, the substance commonly called aspirin. Aspirin can be made by reacting the carboxyl (—COOH) group in acetic acid with the —OH group in the salicylic acid molecule. Follow the O-C7H5O2 portion of the salicylic acid molecule at the left of the arrow to the product molecule on the right. Acetic Acid O—H CH3—C O + Salicylic Acid H—O—C7H5O2 (Note: salicylic acid has both an acid and an alcohol group on the molecule) (See full structures on next page) Acetylsalicylic Acid O—C7H5O2 CH3—C O + H2O Water A better preparative method, which we will use in this experiment, employs acetic anhydride (structure shown on next page) in the react­ion instead of acetic acid. The anhydride can be considered to be the product of a reaction in which two acetic acid molecules combine, with the elimination of a molecule of water. One half of the acetic anhydride will attach to the alcohol group on the salicylic acid while the other half picks up the hydrogen from the alcohol group. This reaction typically gives excellent yields in a short period of time. A catalyst, normally sulfuric or phosphoric acid, is also used to speed up the reaction. The aspirin you will prepare in this experiment is relatively impure and definitely should not be taken internally, even if the experiment gives you a headache. O O CH3—C O O HO + C HO— HO H3PO4 CH3—C O Acetic anhydride: Two acetic acid molecules condensed together C O— + CH3—C O OH CH3—C Salicylic acid: The top group is a carboxylic acid group; the side group is an alcohol group 2 Experiment Supplies • From the cart 1-50 ml flask 1 Büchner funnel and filter flask 1 filter paper • From your drawer 1-250 ml beaker 1-10 ml graduated cylinder 1 thermometer 1. Weigh 2.0 g of salicylic acid and put it into a 50 ml Erlenmeyer flask. Using a 10 ml graduated cylinder, measure 5 ml of acetic anhydride and pour it into the flask in such a way as to wash down any crystals of salicylic acid on the walls. Add 5 drops of 85 percent phosphoric acid to serve as a catalyst. Caution: Both acetic anhydride and phosphoric acid are reactive chemicals which can give you a bad chemical burn, so use due caution in handling them. If you get any of either on your hands or clothes, wash thoroughly with soap and water. 2. Clamp the flask in place in a 250 ml beaker of water supported on a wire gauze on a ring stand, as shown in the diagram. Heat the water with a Bunsen burner to about 75°C, stirring the liquid in the flask occasionally with a stirring rod. Maintain this temperature for about 15 minutes, by which time the reaction should be complete. Slowly add 2 ml of water to the flask to decompose any excess acetic anhydride. There may be some hot acetic acid vapor evolved for O Acetylsalicylic acid: One half of the acetic anhydride molcule has attached to the alcohol group on salicylic acid The other half of the acetic anydride molecule picks up the H from the alcohol group on the salicylic acid a few seconds as a result of the decomposition, or you may see nothing happen, depending on the amount of excess. 3. When the liquid has stopped giving off vapors, remove the flask from the water bath and add 20 ml of water. Let the flask cool for a few minutes in the air, during which time crystals of aspirin should begin to form. Set the flask in a water/ice mix contained in the bottom half of the 250 ml beaker. This hastens crystallization and increases the yield of the product. When the reaction mix is at ice temperature, proceed to step 4. If crystals are slow to appear, it may be helpful to scratch the inside of the flask with 50 ml a stirring rod. flask 4. Collect the aspirin by filtering the cold liquid through a Büchner funnel using suction. See the Büchner Funnel discussion on page 99. Disconnect the suction and pour the filtrate 250 mll beaker from the collection flask back into the reaction flask. Stir to loosen any crystals sticking to the side of the flask, and then quickly pour back into the funnel. Repeat if necessary, until all the crystals are transferred into the funnel. Disconnect the suction and rinse the crystals with 5 ml of ice-cold distilled water. Let the rinse water sit for about 15 seconds before connecting back to the suction hose. When filtration is complete, draw air through the funnel for a few minutes to help dry the crystals and then transfer them to a double thickness of paper towel. Spread out the crystals in a thin layer on the paper towel, forming a 6 x 6 inch square. Allow the crystals to dry while you calculate the theoretical yield of the aspirin. Note: To calculate the theoretical yield of the aspirin, refer to the chemical equation for its formation shown on page 1. Salicylic acid is the limiting reagent. (Looking at the structure on page 2, any corner not showing an attached group on the benzene ring has a hydrogen atom bonded but not shown. You would need to know this if you were calculating the molecular mass from that structure.) Given that one mole of salicylic acid produces one mole of aspirin, use the mass of salicylic acid and the molecular masses of the two substances to calculate the number of grams of aspirin that could be produced from the salicylic acid. Transfer the aspirin to one of the containers provided. Use a folded piece of weighing paper to help transfer the crystals. Use a paper towel to catch any spills. Label the container as follows (use a fine point black ink pen): acetylsalicylic acid 3 mass your name DAT A Mass of salicylic acid used __________g Molecular mass of salicylic acid __________g·mol-1 Moles of salicylic acid used (show calculation) __________mol Molecular mass of acetylsalicylic acid __________g·mol-1 Theoretical yield of acetylsalicylic acid (show calculation) __________g Actual yield __________g % of theoretical yield actual x 100 theoretical __________% date Show the instructor the completed questions, the data sheet and the correctly labeled container to receive a grade. Take the aspirin home to save as a memento, not to use. Commercially prepared aspirin would be purified by recrystallization before it would pass FDA regulations. The sample you have prepared contains a small percent of unreacted salicylic acid as well as very small quantities of the ester formed when the alcohol on one salicylic acid molecule reacts with the acid group on another salicylic acid molecule. Commercial aspirin tablets contain 325 mg per tablet. How many tablets would your yield make? _______ Name__________________________________________________________ Grade___________ Date ___________ Questions 1. It is known that when an ester forms, the OH group from the acid reacts with the H atom on the alcohol to form water, rather than the OH group from the alcohol reacting with the H atom from the acid. How do you suppose they could tell one oxygen from another? (Hint: Can oxygen atoms have different masses?) 2. Show the esterification reaction of methanol, CH3OH, with the acid group on salicylic acid. The acid group is the group at the top of the molecule on page 2. Draw the structure of the salicylic acid molecule as it appears on page 2. 4 3. Show the esterification reaction of methanol with acetic acid and with acetic anhydride. For the reaction with acetic acid, use the generic reaction on page 1. R and R' are both CH3 for these two molecules. Use the reaction shown on page 2 as a template for the acetic anhydride reaction. Replace all but the HO at the 9 o'clock position on the salicylic acid molecule with a CH3 to make the drawing become methanol. Do the same on the right side of the arrow.