Chapter 9 Notes
advertisement
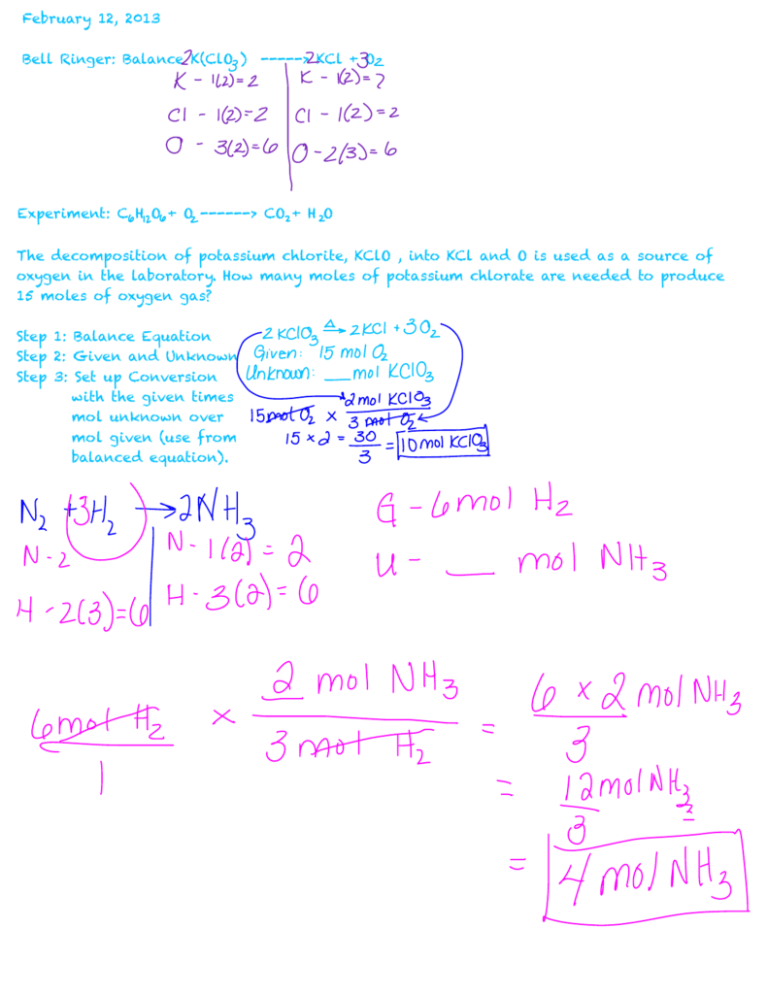
February 12, 2013 Bell Ringer: Balance K(ClO ) -----> KCl + O Experiment: C H O + O ------> CO + H O The decomposition of potassium chlorite, KClO , into KCl and O is used as a source of oxygen in the laboratory. How many moles of potassium chlorate are needed to produce 15 moles of oxygen gas? Step 1: Balance Equation Step 2: Given and Unknown Step 3: Set up Conversion with the given times mol unknown over mol given (use from balanced equation). Hydrogen and oxygen react under a specific set of conditions to produce water according to the following: 2H + O -----> 2H O . A.) how many moles of hydrogen would be required to produce 5.0 mols of water? B.) how many moles of oxygen would be required. 6a.) If 4.50 mol of ethane C H undergo combustion according to the unbalanced equation C H + O -------> CO + H O , how many mol of oxygen is required? b.) How many moles of each product are produced? February 13, 2013 Practiced Additional Examples. February 14, 2013 Solving Gram to Gram Stoichiometry Problems Steps to Solve: 1.) Balance equation 2.) Identify your Unknown and Your Given 3.) Find the Molar Mass of Your Given and Unknown. 4.) Complete your Conversion with the following Equation. Example 2: Tin II Fluoride (SnF ) is used in some toothpastes. It is made by the following reaction : Sn + HF -------> SnF + H . How many grams of SnF are produced when 30.0 grams of HF are consumed? February 15, 2013 Bell Ringer : Get out g-g notes and finish the 2nd example. Practice Gram to Gram Notes 1.) Laughing gas (nitrous oxide N O) is sometimes used as an anesthetic in dentistry. It is produced when ammonium nitrate is decomposed according to the following reaction. NH NO -------> N O + 2H O When copper metal is added to silver nitrate in solution, silver metal is and copper (II) nitrate are produced. What mass of silver is produced from 100.0 g of Cu? February 20-22,2013 Limiting reactants Step 1: Write out equation and Balance the equation. Step 2: List givens and unknowns Step 3: Gram to Gram (2 separate problems) Step 4: Find the limiting and excess reactants. Step 5: Find how much excess reactant is there. (Another gram-gram problem) Step 6: Subtract Your original from the used to find excess. 1.) Zinc and Sulfur react for form zinc sulfide according to the following equation: 8Zn + S -------> 8ZnS A.) If 2 mol of Zn are heated with 1.00 mol of S , identify the limiting reactant. B.) How many moles of excess reactant remain? C.) How many moles of the product are formed? Pg 321 #26 and paragraph 26.) Sulfuric Acid reacts with Aluminum Hydroxide by a Double Replacement. A.) If 30.0g of sulfuric acid react with 25.0g of aluminum hydroxide, identify the limiting reactant. B.) Determine the mass of excess reactant remaining. C.) Determine the mass of each product form. Assume 100% yield. February 28, 2013 Percent Yield Bell Ringer: What does YIELD mean? Actual Yield - measured amount of product obtained by a reactant Percent Yield - the ratio of the actual yield to the theoretical yield multiplied by 100. Example: N + H ----> NH A.) if 13.0g N is reacted, what is the theoretical yield of NH ? B.) If the actual yield is 14.9 g of NH , what was the percent yield? C.) skipped! D.) why might the yield be low? Example 2: C H + Cl -------> C H Cl + HCl a.) If 10.0 g of C H is reacted, what is the theoretical yield of C H Cl? b.) If the actual yield is 15.1g of C H Cl, what was the percent yield? C.) SKIPPED! d.) Why might the yield be high? March 1, 2013 bell ringer H + O -------> H O a.) If 3.0 g of H is reacted, but 17.9g of H O is produced, what is the percent yield? Percent Error: How far away is the yield from 100%. a.) 85% yield = ________ % error b.) 107% yield = ________ % error Practice #1 : Methanol can be produced through the reaction of CO and H in the presence of a catalyst. If 75.0g of CO reacts to produce 68.4g of CH OH, what is the percent yield? CO + H ---------> CH OH









