Chapter 9 Molecular Geometry
advertisement

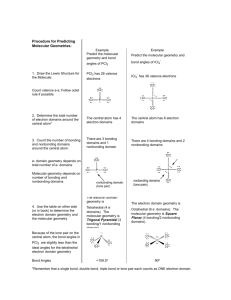
From 2-D to 3-D Chapter 9 Molecular Geometry 1 1 Moving on to Shapes • A chemical formula tells us The identity of atoms The number of atoms • The Lewis structure tells us The identity of atoms The number of atoms How the atoms and electrons are arranged. • The molecular geometry tells us The identity of atoms The number of atoms How the atoms and electrons are arranged The 3-d shape of that atom arrangement. 2 2 VSEPR Model • Valence Shell Electron Pair Repulsion • A fancy name for a simple model. • A model used to sort out how the bonding and nonbonding electrons orient themselves in three dimensions In the model, pairs of electrons arrange themselves to get as far away from each other as possible in order to minimize their negative repulsions. 3 3 Vocabulary • Electron Domain a region around a central atom where electrons are likely to be located. Bonding pair - a pair of electrons between two nuclei. Unshared pair (nonbonding pair, lone pair) - a pair of electrons Multiple bonds - double or triple bonds make up a single domain. • Consider O3 three domains around the central oxygen. Bonding pair Unshared pair Multiple bond 4 4 Electron Domain Geometry • 2 domains • Build it with a styro-ball and 2 toothpicks • Linear • Angle 180º 5 5 Electron Domain Geometry • • • • 3 domains Build it with styro-ball and 3 toothpicks Trigonal Planar Angles 120º 6 6 Electron Domain Geometry • 4 domains • Build it with styro-ball and 4 toothpicks • Build it with the model kit. Find a ball with 4 holes. • Tetrahedral • Angles 109.5º 7 7 Electron Domain Geometry • 5 domains • Build it with your model kit. Use the ball with 5 holes. • Trigonal bipyramidal • Angles 120º 90º 180º 8 8 Electron Domain Geometry • 6 domains • Build it with the model kit. Find the ball with 6 holes. • Octahedral • Angles 90º 9 9 Molecular Geometry What is the shape of the atoms? • AB2 Example: CO2 SCN− C 2H 2 • 2 electron domains Linear domains • 0 nonbonding domains • Linear molecule • Bond Angles 180º 10 10 Molecular Geometry What is the shape of the atoms? • AB3 Examples BF3 CH2O NO3− (CH3)2CO • 3 electron domains Trigonal planar • 0 nonbonding domains • Trigonal planar molecule • Bond Angles 120º 11 11 Molecular Geometry What is the shape of the atoms? • AB2 Examples: O3 NO2− • 3 electron domains Trigonal planar • 1 nonbonding domain • Bent molecule • Bond Angles < 120º 12 12 Ozone • O3 • 3 domains = trigonal planar domains • 1 unshared pair = bent molecule 13 13 Molecular Geometry What is the shape of the atoms? • AB4 Examples CH4 CF2H2 • 4 electron domains Tetrahedral • 0 nonbonding domain • Tetrahedral molecule • Bond Angles 109.5º 14 14 Molecular Geometry What is the shape of the atoms? • AB3 Examples NH3 PCl3 • 4 electron domains Tetrahedral • 1 nonbonding domain • Trigonal pyramidal molecule • Bond Angles < 109.5º 15 15 Molecular Geometry What is the shape of the atoms? • AB2 Examples H 2O CH3OH • 4 electron domains Tetrahedral • 2 nonbonding domain • Bent molecule • Bond Angles << 109.5º 16 16 Bond Angles • Resketch the molecular shapes of CH4, NH3, and H2O. • Consider the bond angles. • Unshared pairs take up more space than bonding pairs. 17 17 More on Bond Angles • Sketch the Lewis structure for CCl2O. • Consider the bond angles. • Multiple bonds bulge and take up more space that single bonds. 18 18 Build a Trigonal Bipyramidal Structure • Consider the five positions different or the same? • There are two different positions in this expanded octet molecule 2 Axial positions 3 Equatorial positions 19 19 Molecular Geometry What is the shape of the atoms? • AB5 Example PCl5 • 5 electron domains Trigonal bipyramidal • 0 nonbonding domain • Name? Trigonal bipyramidal molecule • Bond Angles 90º, 120º, 180º 20 20 Molecular Geometry What is the shape of the atoms? • AB4 Example SF4 • 5 electron domains Trigonal bipyramidal • 1 nonbonding domain • Name? See saw molecule • Bond Angles < 90º, < 120º, < 180º 21 21 Molecular Geometry What is the shape of the atoms? • AB2 Example ClF3 • 5 electron domains Trigonal bipyramidal • 2 nonbonding domains • Name? T-shape molecule • Bond Angles << 90º, << 180º 22 22 Molecular Geometry What is the shape of the atoms? • AB2 Examples XeF2 I3-1 • 5 electron domains Trigonal bipyramidal • 3 nonbonding domains • Name? Linear molecule • Bond Angles 180º 23 23 Molecular Geometry What is the shape of the atoms? • AB6 Example SF6 • 6 electron domains Octahedral • 0 nonbonding domain • Name? Octahedral molecule • Bond Angles 90º, 180º 24 24 Molecular Geometry What is the shape of the atoms? • AB5 Example BrF5 • 6 electron domains Octahedral • 1 nonbonding domain • Name? Square pyramidal molecule • Bond Angles < 90º 25 25 Molecular Geometry What is the shape of the atoms? • AB4 Example XeF4 • 6 electron domains Octahedral • 2 nonbonding domains • Name? Square planar molecule • Bond Angles 90º, 180º 26 26