Experiment 21 Lewis structures and VSEPR Theory
advertisement

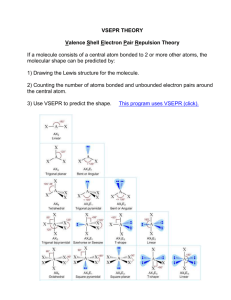
Experiment 21 Lewis structures and VSEPR Theory Introduction 1. Lewis Structures and Formal Charge LG.N. Lewis, at the University of California at Berkeley devised a simple way to understand the nature of the chemical bond in both ionic and molecular compounds. His method rests upon focusing on the valence electrons, i.e. on the electrons in the outer most energy level of an atom or ion. He represents these valence electrons as "dots" around the four sides of the elemental symbol as shown in the figure below. Lewis structures of molecules or ionic species are representations showing all electrons (bonding and nonbonding)of the each element of the compound. The bonding or shared electron pairs are shown either as lines or pair of dots between two atoms. The nonbonding or lone pairs are shown as pair of dots on individual atoms. Atoms, other than hydrogen, tend to gain or lose, or share electrons until it is surrounded by eight valence electrons satisfying the rule called OCTET RULE. This is the electron arrangement corresponding to the configuration of a noble gas (ns2np6). Here are the rules to write the Lewis structures for ionic or molecular compounds: 1. Draw skeletal structure of compound showing what atoms are bonded to each other. 2. Put the least electronegative element in the center (H and F are usually in terminal positions). 3. Count total number of valence e- and add 1 e- for each negative charge or subtract 1 e- for each positive charge. 4. Draw single covalent bonds (2 electrons) between all the atoms and complete an octet for all atoms except for the central atom. 5. Complete an octet for the central atom and, if the octet rule is not satisfied, add multiple bonds on central atom as needed (see some examples below). 57 Sometimes we can write more than one Lewis structure for certain molecules or polyatomic ions. It is possible to determine the correct lewis structures using the concept of formal charge. The formal charge of an atom is the charge that an atom (in a molecule) would have if all of the atoms had the same electronegativity and is calculated using the following equation: Formal Charge = (# of valence e-) – (# of nonbonding e-)– ½ (# of bonding e-) So the best Lewis structure is the one with the fewest formal charges and that puts a negative charge on the most electronegative atom. For example: F. C. = -2 0 +1 -2 0 +1 0 0 -1 best Lewis structure 2. VSEPR and Hybridization Theory Lewis structure provides only the position of the atoms within the molecule, the number of bonding and nonbonding electrons and the type of bonds. Valence Shell Electron Pair Repulsion model (VSEPR) provides a method to predict the shape of most compounds. The theory works on the principle that electron pairs in the valence shell repel each other and thus will remain as far from each other as possible The rules of this model are laid out in the table below, with E representing a cental atom and X representing bonded atoms: 58 Notice that the bonded atoms will attempt to fill the empty 3D space around a central atom evenly. As lone pairs of electrons are introduced, the electron pairs will repel the atoms. If more than one lone pair is present the lone pairs will repel each other first, and the overall shape of the molecule is governed by the number of lone pairs. The bonded atoms will evenly fill up the molecule wherever they may reach an equilibrium of repulsion between the lone pairs. Let’s finally discuss how the the bonds between the atoms are formed so that the molecules can assume the shape described in the VSEPR theory. Hybridiation is a process in which atomic orbitals are mixed to form new identical orbitals and is useful in the explanation of the shape of both ionic species and molecules. The concept of hybridization is an integral part of valence bond theory. In the valence bond theory the bonds are assumed to be formed by overlap of atomic orbitals and the shared region in space between orbitals is the orbital overlap. There are two e- (one from each atom) with opposite spin in the orbital overlap. Linus Pauling first developed the hybridization theory in order to explain the structure of molecules such as methane (CH4). The hybrids are named based on the atomic orbitals that are involved in the hybridization, and the geometries of the hybrids are also reflective of those of the atomic-orbital contributors. For example, in the methane (CH4) a set of sp3 orbitals are formed by mixing one s and three p orbitals on the carbon atom, and are directed towards the four hydrogen atoms which are located at the vertices of a regular tetrahedron as shown in the scheme below: 59 Here are listed the steps necessary to predict the hybridization of the central atom 1. Draw a Lewis structure 2. Count the number of bonding pairs and the number of nonbonding pairs 3. Assign the electron-domain geometry using VSEPR theory 4. Specify the hybridization required to accommodate the bonding and nonbonding electron pairs based on their geometric arrangement according to the following table: Class AB2 AB3 AB4 AB5 AB6 Hybridization sp sp2 sp3 sp3d sp3d2 Electron Domain Geometry linear trigonal planar tetrahedral trigonal bipyramidal octahedral Where A represents the central atom and B the electron domains surrounding the central atom. Procedure Before attending the lab you will focus in assigning the Lewis structure and the formal charge of the molecules and ions litsted in the table below, according to the rules described in the introduction. During the lab period you will determine the VSEPR geometry of each species the hybridization of the central atom, and the overall dipole moment and when possible its orientation within the molecule or ion listed in the worksheet. Visualizing these shapes is not always easy, and 3D models can help in this aspect. In the lab, you will be gprovided with molecular modeling sets to build the molecules. The modeling pieces are a useful tool because they will allow you to see whether or not the dgeometry you have drawn will work within the VSEPR rules. Build a model of every molecule after you have filled out it’s row in the table; if the model will not fit together this is a warning flag that the geometry is incorrect. The 3D model will also be useful to help visualize 3D symmetry when checking for a net dipole in the molecule. Also, building a 3D model will help you to interlalize these visualizations, If you are having a hard time with the VSEPR diagram, try piecing together a 3D model (or models) of what you have so far, and this may help you to see what comes next. On each row in the worksheets, you will be given a compound. As you work through the columns you will be guided toward the VSEPR diagram by working out the preliminary information needed. Refer to the tables above as well as your book and lecture notes to draw the diagram. Remember that this lab is to be done individually and all work must be your own. 60 Worksheets: Formula and # of assigned valence eletrons Lewis Structure Central atom(s) (CAs) Formal charge on each atom 1. # of lone pairs 2. # of electron domains on CA(s) Bond angles around CA(s) H2O NH3 CH4 CO2 NH4+ H3O+ 61 1.Electron domain geometry 2. Molecular geometry VSEPR structure with approximately correct bond angles Dipole moment and orientati on Hybrid. of CA(s) Formula and # of assigned valence eletrons Lewis Structure Central atom(s) (CAs) Formal charge on each atom 1. # of lone pairs 2. # of electron domains on CA(s) Bond angles around CA(s) N2H4 H2O2 HCN CH3OH CH3COOH SO2 62 1.Electron domain geometry 2. Molecular geometry VSEPR structure with approximately correct bond angles Dipole moment and orientation Hybrid. of CA(s) Formula and # of assigned valence eletrons Lewis Structure Central atom(s) (CAs) Formal charge on each atom 1. # of lone pairs 2. # of electron domains on CA(s) Bond angles around CA(s) PCl5 SF4 ClF3 XeF2 SF6 ICl2- 63 1.Electron domain geometry 2. Molecular geometry VSEPR structure with approximately correct bond angles Dipole moment and orientation Hybrid. of CA(s) Formula and # of assigned valence eletrons Lewis Structure Central atom(s) (CAs) Formal charge on each atom 1. # of lone pairs 2. # of electron domains on CA(s) Bond angles around CA(s) BrF5 XeF4 H2SO4 SO42- IF5 ICl4- 64 1.Electron domain geometry 2. Molecular geometry VSEPR structure with approximately correct bond angles Dipole moment and orientation Hybrid. of CA(s)