Vacuolar protein sorting mechanisms in plants
advertisement

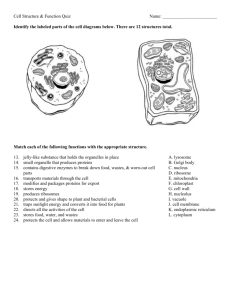
REVIEW ARTICLE Vacuolar protein sorting mechanisms in plants Li Xiang1, Ed Etxeberria2 and Wim Van den Ende1 1 Laboratory of Molecular Plant Biology, KU Leuven, Belgium 2 Horticulture Department, Citrus Research and Education Center, University of Florida, Lake Alfred, FL, USA Keywords adaptor protein complex; BP80; endomembrane; ER body; Golgi; protein storage vacuole; signal peptide; sorting; trafficking Correspondence W. Van den Ende, Laboratory of Molecular Plant Biology, KU Leuven, Kasteelpark Arenberg 31, Leuven, Belgium Fax: +32 16321967 Tel: +32 16321952 E-mail: wim.vandenende@bio.kuleuven.be (Received 14 July 2012, revised 8 November 2012, accepted 11 December 2012) Plant vacuoles are unique, multifunctional organelles among eukaryotes. Considerable new insights in plant vacuolar protein sorting have been obtained recently. The basic machinery of protein export from the endoplasmic reticulum to the Golgi and the classical route to the lytic vacuole and the protein storage vacuole shows many similarities to vacuolar/lysosomal sorting in other eukaryotes. However, as a result of its unique functions in plant defence and as a storage compartment, some plant-specific entities and sorting determinants appear to exist. The alternative postGolgi route, as found in animals and yeast, probably exists in plants as well. Likely, adaptor protein complex 3 fulfils a central role in this route. A Golgi-independent route involving plant-specific endoplasmic reticulum bodies appears to provide sedentary organisms such as plants with extra flexibility to cope with changing environmental conditions. doi:10.1111/febs.12092 Introduction A typical plant or animal cell contains up to 10 000 different types of proteins, whereas a yeast cell contains approximately 5000. For proper functioning, each of these numerous proteins must be localized to a precise intracellular compartment, cellular membrane or organelle, or directed to the exterior of the cell [1]. In plants, the endomembrane system is a complex network of organelles specialized in the synthesis, transport, modification and secretion of proteins and other macromolecules. This system is composed of several functionally distinct membrane compartments: the endoplasmic reticulum (ER), the Golgi apparatus including the trans-Golgi network (TGN), secretory vesicles, the vacuole and endosomes [2]. The mem- branes of mitochondria and chloroplasts do not belong to the endomembrane system [1–3]. Connection between the various endosomal compartments is achieved through tightly controlled, constant budding and fusion of vesicle shuttles [4]. The endomembrane system integrates several dynamic routes: the secretory pathway (including biosynthesis and sorting) and the endocytic pathway. As a result of their overlapping routes and cargo distribution centres, it is difficult, if not impractical, to separate these pathways from each other. Proteins and other cargos are synthesized and programmed to follow a certain sorting pathway to reach their final destination. Although the dynamic activities of endomembrane systems are highly Abbreviations AP, adaptor protein; Arf, ADP ribosylation factor; CCV, clathrin-coated vesicle; COP, coat protein complex; DV, dense vesicle; ER, endoplasmic reticulum; LV, lytic vacuole; MPR, mannose 6-phosphate receptor; PSV, protein storage vacuole; RMR, receptor membrane ring-H2; Sar, secretion-associated RAS-related protein; SNARE, soluble nethylmaleimide sensitive factor attachment protein receptor; TGN, trans-Golgi network; TIP, tonoplast intrinsic protein; VSD, vacuolar sorting determinant; VSR, vacuolar sorting receptor. FEBS Journal 280 (2013) 979–993 ª 2012 The Authors Journal compilation ª 2012 FEBS 979 L. Xiang et al. Vacuolar protein sorting in plant cells conserved among all eukaryotes, higher plants have developed some unique mechanisms [5]. In plants, the secretory and biosynthetic trafficking pathways are involved in a series of vital mechanisms, such as gravitropism, autophagy, hormone transport, cytokinesis and abiotic/biotic stress responses [6,7], as well as in ion secretion by salt glands, nectar production, and the secretion of viscin, which is the elastic, mucilaginous and sticky tissue that attaches falling parasitic mistletoe seeds to branches [8]. Over recent years, remarkable progress has been achieved in the understanding of plant protein and membrane trafficking by the use of different systems and approaches [4,9]. The path followed by a protein depends on the interactions between sorting motifs present in the protein and the motif-recognizing machinery. Many of these motifs are universally conserved among eukaryotes (yeast and humans) [9]. However, sorting motif studies in plants are still in their infancy compared to animal and yeast systems. In plants, studies mainly address tissue-specific and organelle-specific trafficking processes [9]. Generally, the protein secretory pathway begins in the ER, passes through the Golgi complex, and finally reaches the vacuole, other compartments or the cell surface [4,9]. This review mainly focuses on the mechanisms involved in protein sorting to the central vacuole as a unique, multifunctional plant-specific organelle. Sorting of proteins to plant vacuoles Vacuolar proteins reach the different types of vacuoles through a vesicle-mediated biosynthetic trafficking pathway that includes the ER, the Golgi apparatus, the TGN and the endosomes/prevacuoles (Fig. 1). In plant cells, two different types of membrane receptors are known: the vacuolar sorting receptor (VSR) family [9] and the receptor membrane ring-H2 (RMR) family [10]. The plant vacuole The number and the size of plant vacuoles depend on cell type and developmental stage. A single central vacuole may occupy as much as 80% of the volume of a cell. Plant vacuoles are essential multifunctional organelles distinct from similar organelles in other eukaryotes. They serve both physical and metabolic functions, and are crucial to processes involved in the cellular responses to environmental and biotic factors, as well as to general cell homeostasis [11,12]. Vacuoles typically store water, ions, secondary metabolites and nutrients. Similar to animal lysosomes, they also act as a repository for waste products, excess solutes and 980 A B Fig. 1. Model of protein sorting pathways to the plant vacuole. (A) Protein sorting to the PSV. Proteins destined for the PSV initiate their life in the ER and can be sorted to the PSV through Golgidependent or -independent pathways. In the Golgi-dependent pathway, proteins are transported to the Golgi via COPII vesicles, and then aggregate and become the cargo of DVs, being transported into multivesicular bodies and then fusing into the PSV. Notably, some storage proteins that need processing in the Golgi reach the PSV via precursor-accumulating vesicles. In the Golgiindependent pathway, PSV residents are packed into precursoraccumulating vesicles and are transported to the PSV. MVB, multivesicular body; PAC, precursor-accumulating vesicle; PV72/ RMR, vacuole sorting receptor. (B) Protein sorting to the LV. Proteins destined for the LV initiate their life in the ER and can be sorted to the LV through Golgi-dependent or -independent pathways. In the Golgi-dependent pathway, the protein can be (a) recognized by BP80/VSR and becoming the cargo of CCV, after which it is transported to the prevacuolar compartment and, finally, to the LV; or (b) transported via a putative AP3-mediated pathway to the LV. In the Golgi-independent pathway, the LV residents are transported through the ER bodies. A PSV (a-TIP) might be transformed into an LV (c-TIP). Fusions of LVs or PSVs make up the central vacuole. PVC, prevacuolar compartment; BP80/VSR, vacuole sorting receptor. toxic substances [13–16], and play key roles in (programmed) cell death [17–20]. Two types of vacuoles can be found in plant cells, the protein storage vacuole (PSV) and the lytic vacuole (LV) [21]. Typically, vacuolar storage proteins accumulate in PSVs. PSVs have higher pHs and lower hydrolytic activities than LVs, and predominate in storage tissues (e.g. cotyledons and endosperm in seeds, tubers), as well as in vegetative tissues of adult plants (e.g. bark, leaves, pods) [22,23]. Proteins stored in PSVs are mainly used as nitrogen or carbon sources during seed germination and plant development [24]. However, PSVs can also contain large amounts of toxic proteins (e.g. lectins, FEBS Journal 280 (2013) 979–993 ª 2012 The Authors Journal compilation ª 2012 FEBS L. Xiang et al. protease inhibitors and ribosome inactivating proteins), which can be considered as the result of a cytosolic detoxification process or as a defence against predators [25–27]. PSV proteins could be processed through the action of vacuolar processing enzymes or proteases [28–30]. By contrast, LVs are usually found in vegetative tissues. They have an acidic pH and contain an abundance of hydrolytic enzymes [31,32]. LVs are used for storage and as a depository of unwanted materials in plant cells. They receive extracellular components via endocytosis and phagocytosis, and intracellular material via autophagy, as well as via the biosynthetic trafficking pathway and membrane-bound transport systems. LVs modulate the degradation of a multitude of macromolecules and other compounds. As such, they are considered key regulators in cellular homeostasis [33,34]. Tonoplast intrinsic proteins (TIPs) have been used as intracellular markers for vacuolar biogenesis and identity. The expression of TIPs, although tissue-specific, varies greatly throughout development. TIPs are classified into five categories: a, b, c, d and e-TIPs [35]. a and b-TIPs are seed-specific. c-TIPs associate with the LV, whereas a-TIP and d-TIP associate with the PSV [36,37]. e-TIPs are primarily found in root and floral organs. Different TIPs can coexist in the same cell, suggesting the presence of both LVs and PSVs [33]. Evidence that LVs and PSVs can occur together has been found in the root tip cells of barley and pea seedlings [31,38], as well as in protoplasts of barley aleurone and tobacco [32]. Interestingly, it was reported that, during the germination of Arabidopsis seeds, the LV is primarily embedded in the PSV and then derives from it, instead of being generated de novo [21,31,39]. Moreover, depending on physiological conditions, LVs can be transformed into PSVs and vice versa [40–42]. In addition to PSVs and LVs, a third type of vacuole has been suggested in Arabidopsis [43,44]. During leaf senescence, senescence-associated vacuoles, with a smaller size and containing aggregates, are formed de novo [45], and are characterized by a higher cysteine-protease activity and a lower pH than LVs [46]. The existence of two types of plant vacuoles with distinct contents and functions implies that separate trafficking pathways must exist for their respective cargo [5], with a need for correct separation in the Golgi/TGN apparatus (Fig. 1). Furthermore, it can be hypothesized that the concurrent existence of LVs and PSVs provides plants with extra flexibilities to deal with changing environmental conditions. Vacuolar protein sorting in plant cells Sorting of vacuolar proteins is initiated in the ER The initiation of vacuolar/lysosomal protein sorting in the ER is a very conserved mechanism in yeast, animals and plants [5,9]. The ER represents the first compartment of the secretory system [47]. The ER membrane connects to the nuclear envelope, and forms a wide network of thin tubules and cisternae in the cortical and inner parts of the cell [47]. Both transmembrane proteins and soluble, secreted proteins contain an ER signal sequence including a hydrophobic 20–25 amino-acid segment. As a result of its hydrophobic nature, the signal sequence is inserted into the ER membrane. The difference between the secreted and transmembrane proteins is that the hydrophobic sequence is removed by the ER signal peptidases from the secreted proteins, although not from the transmembrane proteins [48,49]. Based on the model of Singer and Nicolson [50], integral membrane proteins can be classified as: (a) type I transmembrane proteins containing a single transmembrane domain with an Nout/Cin orientation (‘in’ means cytoplasmic side, ‘out’ means lumen); (b) type II transmembrane proteins containing a single transmembrane domain with an Nin/Cout orientation; (c) type III membrane proteins (multiple transmembrane domains in a single polypeptide chain); and (d) type IV membrane proteins (multiple transmembrane domains reside on a single or multiple individual polypeptide chain). The ER is also an important check point of correct protein folding and assembly, known as stringent quality control [51]. Only properly folded and assembled proteins are allowed to exit the ER. Misfolded proteins are recognized by molecular chaperones (e.g. BiP, calnexin) and retained in the lumen of the ER in an attempt to re-fold them to their correct, native structure [52–54]. Persistent misfolded proteins are transferred to the cytosol and degraded by the proteasome system [55,56]. Proteins that have erroneously reached the Golgi can also return to the ER when ER retention signals are present [57]. Vesicular trafficking Plant cells, similar to all eukaryotes, are structurally and metabolically subdivided into different compartments, with communication between organelles being accomplished mainly by vesicular trafficking. This is a highly-regulated and directional/targeted process. In general, the vesicle trafficking process involves budding, vesicle release, targeted transport, tether, and membrane recognition and fusion [58]. Vesicle trafficking is also involved in secretion into the apoplast and, FEBS Journal 280 (2013) 979–993 ª 2012 The Authors Journal compilation ª 2012 FEBS 981 L. Xiang et al. Vacuolar protein sorting in plant cells during endocytosis, recycling proteins from the plasma membrane to the endosome, the TGN and the lysosome/vacuole [5,47]. In all eukaryotes, clathrin-coated vesicle (CCV), caveolin, coat protein complex I (COPI) and coat protein complex II (COPII) vesicles carry out these functions and have an ubiquitous presence and budding machineries. Vesicle formation is not a default, passive event, but instead, requires a specific driving force to carry out a series of highly-regulated events including recognition and binding between the receptor at the donor membrane and an activated GTP-ADP ribosylation factor (Arf) complexed with the secretion-associated RASrelated protein (Sar)1 complex, recruiting coatomer, membrane distortion and dissociation under the assistance of fatty acyl CoA, and the formation and release of a vesicle. Similar to animals and yeast, in plants, most of the Sar/Arf1 GTPases are well conserved. The Arf1 family is necessary for COPI vesicle and CCV formation, whereas the Sar1 family is mainly involved in COPII vesicle formation [59,60]. After formation, the vesicle is released and travels towards the target compartment assisted by actin filaments, where it disassembles with the help of a cytosolic Hsc70 chaperone. After the uncoating process [61–63], specific vesicle soluble nethylmaleimide sensitive factor attachment protein receptor (SNARE) proteins, exposed on the vesicle’s membrane, bind to cognate targetSNARE proteins complexed with synaptosomal-associated protein 25. Next, nethylmaleimide sensitive factor and soluble nethylmaleimide sensitive factor attachment proteins bind to this complex, which dissociates after vesicle fusion. Sec1p and Rab proteins serve as effectors for SNARE complex regulation during vesicle targeting and fusion [62–64]. Trafficking between the ER and the Golgi apparatus Although the sorting of plant vacuolar proteins commences at the ER [64], the Golgi apparatus is the major sorting station in the plant cell [65]. The ER and Golgi communicate with each other via a highlyregulated traffic system. Transfer from the ER to the Golgi apparatus is the first step in protein sorting via the biosynthetic trafficking pathway. This is mediated by COPII vesicles. The retrograde transport from the Golgi to the ER is accomplished via COPI vesicles, which are morphologically and biochemically different from the COPII vesicles of the retrograde system [66,67]. The COPII vesicles mediate the anterograde traffic export protein from ER the to the Golgi apparatus in eukaryotic cells [68,69]. COPII is composed of three 982 components: Sar1, Sec23/24 and Sec13/31. After budding off from the ER, COPII vesicles can either directly fuse with cis-Golgi, or first fuse with each other and then to the cis-Golgi [68,70,71]. The role of COPI vesicles for retrograde traffic from the Golgi to the ER or within the Golgi apparatus from the trans- towards the cis-Golgi in plants was first demonstrated in transgenic tobacco [72]. COPI consists of coatomer (F-COP and B-COP subunit) and Arf G-protein. Two classes can be discerned: COPIa vesicles are derived from cis-cisternae, and COPIb vesicles are derived from medial and trans-cisternae [73]. Different classes of COPI vesicles are caused by multiple isoforms of COP subunits [73]. The distribution of proteins between ER and Golgi is maintained by the balanced cooperation of COPI and COPII transport routes. Inhibition of COPI function results in impaired trafficking between the Golgi and the ER and disruption of the ER export sites [72,74,75]. For exit of proteins from the ER, different types of motifs have been identified that are recognized by COPII. Di-acidic (e.g. DXE/EXE), di-basic (e.g. RKXRK) and di-aromatic motifs in the cytosolic tail of transmembrane proteins were reported to be important for the export of proteins from the ER in yeast and animals [68]. The first evidence for a di-acidic motif (DAE) in protein export from the ER in plants was obtained for a sugar transporter in tobacco [76]. Next, Schoberer et al. [77] demonstrated the importance of a di-basic motif (RKR) in tobacco as well. Retrograde Golgi-ER transport of soluble proteins is achieved by C-terminal H/KDEL motifs that are recognized by the receptor ER retention defective 2 protein embedded in the membrane. Thus, H/KDEL motifs are considered to be responsible for the retention of soluble proteins in the ER lumen, and are named ER retention signals. H/KDEL containing proteins never undergo the typical modifications observed in Golgi-derived enzymes [78,79]. Intra-Golgi and post-Golgi transport The plant Golgi apparatus appears to be more complicated than its animal counterpart. It is divided into functionally independent individual Golgi stacks and has a polarized structure, whereas the animal Golgi apparatus is perinuclear and stationary [75]. After receiving newly-synthesized proteins from the ER, the Golgi apparatus performs covalent modifications, and then further distributes the proteins to various final destinations [80].The cis-Golgi constitutes the entrance to the apparatus, whereas vesicles leave at the transGolgi to reach their final destination [65,81]. The FEBS Journal 280 (2013) 979–993 ª 2012 The Authors Journal compilation ª 2012 FEBS L. Xiang et al. Golgi is also implicated in protein trafficking to nonsecretory organelles such as peroxisomes and chloroplasts. The Golgi apparatus plays an important role in post-translational modification mainly through transmembrane processing enzymes. Proteins enter the cis-Golgi, move through the med-Golgi and reach the trans-Golgi where the modification process is completed, such as glycosylation, sulfation and phosphorylation [82]. Two models of intra-Golgi transport have been proposed: the vesicle shuttle and the cisternal maturation models. Notably, multiple Golgi-independent protein transport pathways exist for delivering cargo molecules from the ER to a variety of destinations, such as celery mannitol dehydrogenase delivery to the extracellular space [83] and barley aspartic protease transport to the vacuole [84]. Post-Golgi vesicle transport: the CCV The CCVs were the first type of coated vesicles to be described in eukaryotes. Typically CCVs are 50– 100 nm in diameter, formed at the plasma membrane and TGN/endosomes and are involved in the trafficking of protein cargo between these organelles [85]. There is evidence for their formation at the lysosomes of animals [86] and the vacuoles of yeast [87], yet there are no confirmed reports of CCV formation in plant vacuoles. They are also implicated to play a role in other processes such as defence responses and cytokinesis. The formation of a clathrin coat requires various adaptor proteins (APs), clathrin and dynamin (a GTPbinding protein, Arf). The clathrin unit is a threelimbed shaped triskeleton, each limb containing one clathrin heavy chain and one light chain [88]. Two other proteins, amphiphysin and synaptojanin, are involved in CCV formation as well. The clathrin coat can be depolymerized by cytosolic Hsc70, and re-used [89–91]. A distinctive characteristic of plant CCVs is their larger size. Capacitance measurements of endocytic events during fluid phase endocytosis in tobacco BY-2 protoplasts estimated the size of vesicles to be a mean of 133 nm [92]. These estimates are similar to those obtained by Gall et al. [93] in turgid guard cells. By contrast, estimates of CCVs in nonplant systems appear to be between 70 and 100 nm [94]. Vacuolar sorting machinery Plant vacuolar sorting determinants (VSDs) VSDs are necessary for correct post-Golgi sorting to the vacuole. Plant cells contain three different categories of VSDs: (a) sequence-specific VSDs; (b) C-terminal VSDs; and (c) protein structure-dependent VSDs Vacuolar protein sorting in plant cells [80,95]. Without these motifs, vacuolar proteins follow the default pathway and are secreted to the surface of the cell, whereas their introduction into a secreted protein could redirect it to the vacuole [96]. In general, N-terminal sequence-specific VSDs and C-terminal VSDs are removed during protein maturation after vacuolar sorting by the action of vacuolar proteases [97]. Sequence-specific VSDs are generally considered to be recognized by VSRs for sorting to the LV, as in the case of barley aleurain and sporamin [98–101]. Functional sequence-specific VSDs (NPIXL/NPIR) were also described in storage protein sorting to the PSV, such as castor 2S albumin and ricin [102–104]. Sequence-specific VSDs function independently of their molecular position, although they are most often situated at the N-terminus of a protein. For example, sporamin type sequence-specific VSDs are still able to direct protein to the vacuole after translocation from the N-terminus to the C-terminus [99,105]. There are some examples of C-terminal and even internal sequence-specific VSDs [106]. In plants, C-terminal VSDs were first discovered in barley lectin and tobacco chitinase [107,108]. The minimal length is four amino acids [103]. Many random C-terminal peptides are sufficient to target a reporter protein to the vacuole. For example, the C-terminal VSD of tobacco chitinase A (GLLVDTM), and the FAEAI and LVAE motifs of barley lectin, are necessary and sufficient for vacuolar targeting [107–109]. Moreover, the IAGF motif from 2S albumin of Passifloraceae, the PLSSILRAFY motif of the b-conglycinin a unit of soybean and the KISIA motif from the 11S albumin of Amaranthus are all functional C-terminal VSDs [106,110,111]. C-terminal VSDs must strictly localize to the C-terminal part of the protein. Moreover, the introduction of C-terminal glycosylation sites or addition of extra alanine stretches at the C-terminus led to cell surface secretion [112,113]. Some proteins even combine a sequence-specific VSD and a C-terminal VSD for dual targeting to the PSV matrix and to globoids [114]. Importantly, random C-terminal VSDs appear to be rather unique to plants. Despite more extensive research on animal systems, only a few cases have been reported in animals [115], where tyrosine motifs (YXXΦ) are more commonly used [116,117]. The physical and structural VSDs are based on protein structure, and can be subdivided in two types. The first type may be composed of multiple internal domains forming a higher-order structure to function as a VSD. This is the case for the 11S globulin legumin from field bean. The second type is formed by the aggregation of seed storage proteins, presumably occurring in the Golgi apparatus. Proproteins are FEBS Journal 280 (2013) 979–993 ª 2012 The Authors Journal compilation ª 2012 FEBS 983 L. Xiang et al. Vacuolar protein sorting in plant cells often more hydrophobic than their mature counterparts and therefore they form aggregates. This nonreceptor-mediated sorting mechanism was reported for seed storage proteins reaching the PSV through dense vesicles (DVs), which are unique vesicles only occurring in plants [106,113,118,119]. VSRs In 1994, the first VSR was identified from a pea cotyledon CCV preparation. The protein was termed BP80 (binding protein 80 kDa) by its ability to bind in vitro the VSD of barley aleurain [120]. VSRs are type I membrane proteins with a cytosolic motif and a large lumenal domain. They are not related to TGN sorting receptors such as the mannose 6-phosphate receptor (MPR) in animals or the vacuolar carboxypeptidase sorting receptor VPS10 in yeast [121,122]. VSRs generally recognize NPIRL-(like) consensus motifs in cargo proteins (mostly present at the N-terminus in cargo), whereas, in turn, VSRs themselves are recognized by AP1 via its YMPL consensus motif at the C-terminus. VSRs are generally considered to mediate protein sorting to LV, as supported by several pieces of evidence. First, VSRs recognize and bind the sequence-specific VSDs present in LV proteins, such as barley aleurain and sporamin [123]. Second, VSRs contain a tyrosine motif for interaction with the AP1 complex l1 subunit and subsequent packing into the CCV [5,124]. Third, Arabidopsis thaliana vacuolar sorting receptor 1, a BP80 homologue from Arabidopsis, interacts with AtEpsinR1, with its animal homologue being involved in CCV-mediated trafficking to lysosomes [125,126]. Fourth, the A. thaliana vacuolar sorting receptor 1 C-terminal cytosolic tail interacts with AtVPS35, a prevacuolar compartment localized retromer from Arabidopsis [127]. Fifth, despite the fact that VSRcargo ligand interaction might be initiated in the ER [64], VSRs are accumulating to a high extent in the prevacuolar compartment and to a lower extent in the TGN [128]. Finally, the pH-dependent ligand binding indicates that the receptor-cargo complex needs to end up in an acidic environment to release its cargo [129]. However, several studies also report interactions between VSRs and VSDs of storage proteins such as 2S albumins in castor bean [10,102]. PV72, a pumpkin homologue of VSR, was identified in the precursoraccumulating compartments that are proposed as intermediates in the transport of storage proteins to the PSV [130,131]. Similar results have been found in sunflower [132] and castor bean [112]. By contrast to the vacuolar sorting receptor BP80, ligand binding to PV72 is calcium-dependent because it is released at a 984 low calcium concentration. Additionally, A. thaliana vacuolar sorting receptor 1 (also termed AtELP1) was shown to play a role in sorting storage proteins in seeds, which also shows calcium-dependence [133]. These results indicate that different isoforms may be involved in different sorting pathways. The recent finding that VSRs could be localized at the plasma membrane suggested an additional role for VSR proteins in mediating protein transport towards the plasma membrane and endocytosis in germinating pollen tubes of lily and tobacco [122]. The RMR protein family The RMR protein family RingH2 was originally discovered as a result of their homology to protease-associated domains in VSRs, indicating their role in binding vacuolar proteins [10]. In vitro experiments showed the capability of RMR to bind to the C-terminal VSD of barley lectin, bean phaseolin and tobacco chitinase, which are then transported to the PSV [134,135]. The RMRs are type I transmembrane proteins containing a typical N-terminal signal peptide, followed by a protease-associated domain and a single transmembrane domain [136]. By contrast to the short cytoplasmic tail of VSRs, plant RMRs contain a long cytoplasmic tail with a typical C3H2C3 RING-H2 domain [10]. One isoform of Arabidopsis, AtRMR1, has been mainly localized in the late Golgi apparatus, DVs and in the PSV of Arabidopsis embryos by the use of immunogold electron microscopy [137]. AtRMR2 was localized in the PSV [138], whereas other RMRs were also found in the PSV of tomatoes [13] and in members of the Brassicaceae [139]. These findings are compatible with the hypothesis of its role as a receptor in protein sorting to the PSV [122]. Recently, it was demonstrated that rice RMR1 associated with an intermediate vacuolar-like compartment related to the PSV [140]. AP complexes AP complexes are heterotetramers mediating the formation of transport vesicles, as well as cargo sorting in all eukaryotes. Most of the research on AP complexes has been devoted to animals and yeast. According to Hirst et al. [141], five distinct AP complexes (AP1– AP5) have been identified in eukaryotes to date. AP complexes are composed of two large subunits (termed a/b1, a/b2, d/b2, e/b4 and ξ/b5, respectively), a medium subunit (l1–l5) and a small subunit (r1–r5) [141 –144]. The c/b1 and a/b2 subunits of AP1 and AP2 bind clathrin via clathrin-binding sites within their FEBS Journal 280 (2013) 979–993 ª 2012 The Authors Journal compilation ª 2012 FEBS L. Xiang et al. hinge domains [145]. Each AP complex operates in distinct organelle localization and performs similar functions. The AP1 complex is involved in CCV formation at the TGN and endosomes, mediating the trafficking between these organelles [146,147]. The AP2 complex contributes to the formation of CCV from the plasma membrane and facilitates clathrin-mediated endocytosis [148,149]. The AP3 complex is involved in the formation of vesicles from TGN/endosomes, and mediates transport to lysosomes/vacuoles [147,150]. The clathrin binding of AP3 is under debate. In Arabidopsis, AP3 subunit loss-of-function mutants implicated AP3 in biogenesis and function of the plant LV [151–153]. The AP4 complex was recently defined as a mediator of the transport of the amyloid precursor protein from the TGN to the endosome [154]. It was suggested to be involved in vesicle formation with or without clathrin [155,156]. The AP5 complex does not associate with clathrin, localizes in late endosomal compartments, and mediates endosomal sorting [144]. In the biosynthetic and endocytic trafficking pathways, AP complexes selectively recognize sorting signals [145]. A number of such sorting signals have been identified in the last decade, such as tyrosine signals (NPXY and YXXФ signals, where X could be any amino acid and Ф is a bulky hydrophobic amino acid), and dileucine signals ([DE]XXXXL[LI] and DXXLL consensus motifs) [147]. AP complexes are known to interact with tyrosine-based sorting signals via their l subunits, although the AP subunits that recognize dileucine-based sorting signals remain unidentified. There is evidence indicating that AP1 binds via its c and r1 subunits [157], whereas AP3 binds via its b unit [145,158]. Protein sorting to the vacuole Protein sorting to the PSV Storage proteins are transported to the PSV via Golgidependent or -independent pathways depending on the cargo protein and plant developmental stage [4,159]. Unlike LVs, the trafficking of storage proteins from the Golgi apparatus into the PSV is mediated by DVs rather than by CCVs [160,161]. DVs are small, uniform vesicles (150–200 nm in diameter) containing intrinsic membrane proteins destined for the PSV, and are characterized by their high density electron-opaque lumenal contents [113]. They were first discovered in common bean [160], and later in other plant species, such as wheat [162], pea [163] and Arabidopsis [137]. Within the cis-Golgi, the accumulation and condensation of storage proteins initiates the formation of DVs. Subsequently, these discrete small vesicles are transferred to the TGN Vacuolar protein sorting in plant cells concomitant with increased density and, finally, they are released from the TGN [80,137]. Mature DVs are not protein coated; they can directly fuse with the PSV or first with multivesicular bodies [13,161,164,165]. Multivesicular bodies contain multiple internal vesicles, are present in all eukaryotes, and are involved in various post-Golgi processes of the biosynthetic trafficking pathway. DVs fuse into the multivesicular bodies where they discharge their contents [166]. Multivesicular bodies are then received by the PSV together with their cargos. Therefore, the Golgi-dependent PSV trafficking pathway could be defined as an ER?Golgi?DV? (multivesicular bodies)?PSV pathway (Fig. 1A). Protein transport to the PSV mainly occurs through aggregation sorting, although receptor-mediated sorting might play a role as well [132]. In this case, the involved VSRs are BP80 homologues (such as PV72, AtELP1) and RMRs [80,112,113,167,168]. Many proteins have been reported that sort to the PSV via DVs, such as legumin, vicilin and sucrose-binding-protein homologue [132,169]. Transport of storage proteins to the PSV can also take an alternative route from the ER bypassing the Golgi, as shown in Fig. 1A. Globulins, the major vacuolar storage proteins in pumpkins, were suggested to reach the PSV via precursor-accumulating compartments, which are much larger (diameter of 200–400 nm) than DVs, and reach the PSV directly from the ER [130,170]. Similar results have been obtained for cysteine proteinases [171,172]. Precursor-accumulating compartments contain unglycosylated precursors of storage proteins, and mediate aggregation sorting. Precursoraccumulating compartments have been found in pumpkin, castor beans and in wheat [130,173,174]. Although directly generated from the ER, precursor-accumulating compartments can accept glycosylated proteins derived from the Golgi during their transport to the PSV [112,130]. The content of precursor-accumulating compartments is incorporated in the lumen of the PSV. The incorporation follows one of two models: (a) fusion between precursor-accumulating compartments and PSV occurs through autophagy [175,176] or (b) by direct membrane fusion [177]. Protein sorting to the LV Different pathways for post-Golgi sorting of proteins to lysosomes/vacuoles have been described for yeast and animals. The pathway through CCVs appears to be conserved among all eukaryotes (plants, yeast, animals). Inside the TGN, specific sorting signals are recognized by TGN membrane localized receptors, recruited into CCVs and transported into LVs/lysosomes [165,178]. In FEBS Journal 280 (2013) 979–993 ª 2012 The Authors Journal compilation ª 2012 FEBS 985 L. Xiang et al. Vacuolar protein sorting in plant cells animal cells, the sorting of acid hydrolases to the lysosome is facilitated by the MPR [179]. MPR-ligand complexes are recruited into CCVs at the TGN. This process is mediated by Golgi-localized, c adaptin earcontaining, Arf-binding proteins and by the AP1 complex through interactions with MPRs tyrosine (YXXФ) and dileucine (LL) motifs at the cytosolic tail [179,180]. In yeast, the sorting and delivery to the LV is very similar to the MPR pathway of animal cells [181,182]. This mechanism is assumed to be used for trafficking to plant LVs as well, although LV proteins interact with VSRs, showing no homology to MPRs [85]. Plant VSRs recognize sequence-specific VSDs (e.g. NPIRL-like consensus motifs) in LV targeted proteins, such as barley aleurain [183,184]. VSRs then interact with AP1 through a tyrosine-based sorting motif (e.g. YMPL) instead of through a dileucine motif as observed in yeast and animals [124]. CCVs, containing cargo-VSR, then bud from the TGN, and discharge their contents after fusion into the prevacuolar compartment [163,166]. As a result of the lower pH of the prevacuolar compartment, ligands dissociate from their receptor, and the receptor is subsequently recycled back to the Golgi apparatus [185–187]. Importantly, an MPR-independent and a VPS10-independent lysosome sorting pathway were found in animal cells and yeast, respectively [188,189]. Recent studies on the sorting of tonoplast transporters in Arabidopsis mesophyll protoplasts suggest a similar route in plants (Fig. 1B) [190]. AP3, but not AP1, appears to fulfill a central role in this pathway. In animals, AP3 can recognize both dileucine and tyrosine motifs, whereas, in yeast, only dileucine signals can be recognized [145,191]. Recent reports suggest that AP3 subunits are involved in the biogenesis, morphology and function of the prevacuolar compartment and vacuoles in plants [151– 153]. Remarkably, and uniquely in plants, LV resident proteins can be transported directly from the ER to the LV by means of ER bodies as intermediate compartments, bypassing the Golgi apparatus, as shown in Fig. 1B [170]. Thus, one of the emerging differences that appears to distinguish plants from other eukaryotes is the plasticity of the ER to form protein-, oil- or rubber-containing subcellular structures best termed ER bodies [192], which either stably accumulate or are transported to the LV. The ER bodies are in most instances spherical, < 1 lm in diameter, and consist of a dense core of a self-assembling or aggregating protein, oil or rubber, and a membrane of ER origin [193]. However, there is some evidence for nonconventional ER trafficking bypassing the Golgi to the lysosome in animal cells [194]. It was proposed that ER bodies in plants can follow a similar path, bypassing 986 the Golgi and directly fusing with LV, as observed under stress conditions [195,196]. Conclusions and perspectives Recent advances in our understanding of the processes involved in the sorting of proteins to the vacuole(s) in plant cells suggest that there are relatively highly-conserved processes among eukaryotes. Most differences are related to the uniqueness of the plant vacuole as a storage compartment, as opposed to the animal lysosome and the yeast vacuole, which function predominantly as hydrolytic compartments. Another distinctive feature is the apparent duplicity and alternative pathways for proteins to reach the lytic and protein storage vacuolar lumen or tonoplast. Furthermore, the variety of chemical substances stored in the vacuole (e.g. protein bodies, resins, gums, latex, sugars, etc.) imposes the acquisition of redundant transport pathways to the vacuole. One aspect of the vesicle-mediated delivery system to the vacuole that awaits clarification is how the vacuoles compensate for the increasing uptake of fluids and added membrane during the vesicle fusion process. At present, there are no indications as to how homeostasis is maintained, although, in yeast, the formation of retrograde CCV has been observed. The alternative postGolgi, AP3-mediated route has been well described in yeast and animals, although further experimental work is required to determine whether this route is also fully active in plants. Furthermore, the regulatory mechanisms of the AP3-mediated and Golgi-independent routes, as well as the cargos, receptors and possible budding factors involved, still remain elusive and require further exploration. The use of fluorescent probes, transgenic plants and new imaging techniques will likely provide us with a clearer understanding in the near future. Acknowledgements Li Xiang and Wim Van den Ende are supported by grants from FWO Vlaanderen. References 1 Vitale A & Galili G (2001) The endomembrane system and the problem of protein sorting. Plant Physiol 125, 115–118. 2 Frigerio L & Hawes C (2008) The endomembrane system: a green perspective. Traffic 9, 1563. 3 Dacks JB, Peden AA & Field MC (2009) Evolution of specificity in the eukaryotic endomembrane system. J Biochem Cell Biol 41, 330–340. FEBS Journal 280 (2013) 979–993 ª 2012 The Authors Journal compilation ª 2012 FEBS L. Xiang et al. 4 Rojo E & Denecke J (2008) What is moving in the secretory pathway of plants? Plant Physiol 147, 1493–1503. 5 J€ urgens G (2004) Membrane trafficking in plants. Annu Rev Cell Dev Biol 20, 481–504. 6 Carter C, Pan S, Zouhar J, Avila EL, Girke T & Raikhel NV (2004) The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unpredicted proteins. Plant Cell 16, 3285–3303. 7 Surpin M & Raikhel N (2004) Traffic jams affect plant development and signal transduction. Nat Rev Mol Cell Biol 5, 100–109. 8 Echeverrıa E (2000) Vesicle-mediated solute transport between the vacuole and the plasma membrane. Plant Physiol 123, 1217–1226. 9 De Marcos Lousa C, Gerschlick DC & Denecke J (2012) Mechanisms and concepts paving the way towards a complete transport cycle of plant vacuolar sorting receptors. Plant Cell 24, 1714–1732. 10 Cao X, Rogers SW, Butler J, Beevers L & Rogers JC (2000) Structural requirements for ligand binding by a probable plant vacuolar sorting receptor. Plant Cell 12, 493–506. 11 Marty F (1999) Plant vacuoles. Plant Cell 11, 587–600. 12 Martinoia E, Maeshima M & Neuhaus HE (2007) Vacuolar transporters and their essential role in plant metabolism. J Exp Bot 58, 83–102. 13 Jiang L, Phillips TE, Rogers SW & Rogers JC (2000) Biogenesis of the protein storage vacuole crystalloid. J Cell Biol 150, 755–770. 14 Bethke PC & Jones RL (2000) Vacuoles and prevacuolar compartments. Curr Opin Plant Biol 3, 469–475. 15 Martinoia E, Massonneau A & Frangne N (2000) Transport processes of solutes across the vacuolar membrane of higher plants. Plant Cell Physiol 41, 1175–1186. 16 Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, Kondo M, Nishimura M & Hara-Nishimura I (2004) A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 305, 855–858. 17 Hara-Nishimura I & Hatsugai N (2011) The role of vacuole in plant cell death. Cell Death Differ 18, 1298–1304. 18 Gadjev I, Stone JM & Gechev TS (2008) Programmed cell death in plants: new insights into redox regulation and the role of hydrogen peroxide. Int Rev Cell Mol Biol 270, 87–144. 19 Guicciardi ME, Leist M & Gores GJ (2004) Lysosomes in cell death. Oncogene 23, 2881–2890. 20 Xiao H, Chen D, Fang Z, Xu J, Sun X, Song S, Liu J & Yang C (2009) Lysosome biogenesis mediated by VPS-18 affects apoptotic cell Vacuolar protein sorting in plant cells 21 22 23 24 25 26 27 28 29 30 31 32 33 FEBS Journal 280 (2013) 979–993 ª 2012 The Authors Journal compilation ª 2012 FEBS degradation in Caenorhabditis elegans. Mol Biol Cell 20, 21–32. Bolte S, Lanquar V, Soler M-N, Beebo A, SatiatJeunema^ıtre B, Bouhidel K & Thomine S (2011) Distinct lytic vacuolar compartments are embedded inside the protein storage vacuole of dry and germinating Arabidopsis thaliana seeds. Plant Cell Physiol 52, 1142–1152. M€ untz K (1998) Deposition of storage proteins. Plant Mol Biol 38, 77–99. Zouhar J, Mu~ noz A & Rojo E (2010) Functional specialization within the vacuolar sorting receptor family: VSR1, VSR3 and VSR4 sort vacuolar storage cargo in seeds and vegetative tissues. Plant J 64, 577–588. Wang J, Li Y, Lo SW, Hillmer S, Sun SSM, Robinson DG & Jiang L (2007) Protein mobilization in germinating mung bean seeds involves vacuolar sorting receptors and multivesicular bodies. Plant Physiol 143, 1628–1639. C^andido EDS, Pinto MFS, Pelegrini PB, Lima TB, Silva ON, Pogue R, Grossi-de-Sa MF & Franco OL (2011) Plant storage proteins with antimicrobial activity: novel insights into plant defense mechanisms. FASEB J 25, 3290–3305. Jørgensen M, Stensballe A & Welinder KG (2011) Extensive post-translational processing of potato tuber storage proteins and vacuolar targeting. FEBS J 278, 4070–4087. Lord JM & Spooner RA (2011) Ricin trafficking in plant and mammalian cells. Toxins 3, 787–801. Otegui MS, Herder R, Schulze J, Jung R & Staehelind LA (2006) The proteolytic processing of seed storage proteins in Arabidopsis embryo cells starts in the multivesicular bodies. Plant Cell 18, 2567–2581. Yamada K, Shimada T, Nishimura M & HaraNishimura I (2005) A VPE family supporting various vacuolar functions in plants. Plysiol Plant 123, 369–375. Nakaune S, Yamada K, Kondo M, Kato T, Tabata S, Nishimura M & Hara-Nishimura I (2005) A vacuolar processing enzyme, deltaVPE, is involved in seed coat formation at the early stage of seed development. Plant Cell 17, 876–887. Olbrich A, Hillmer S, Hinz G, Oliviusson P & Robinson DG (2007) Newly formed vacuoles in root meristems of barley and pea seedlings have characteristics of both protein storage and lytic vacuoles. Plant Physiol 145, 1383–1394. Park M, Kim SJ, Vitale A & Hwang I (2004) Identification of the protein storage vacuole and protein targeting to the vacuole in leaf cells of three plant species. Plant Physiol 134, 625–639. Frigerio L, Hinz G & Robinson DG (2008) Multiple vacuoles in plant cells: rule or exception? Traffic 10, 1564–1570. 987 L. Xiang et al. Vacuolar protein sorting in plant cells 34 Rogers JC (2008) Multiple vacuoles in plant cells – response. Plant Physiol 146, 1024–1025. 35 Maurel C (2007) Plant aquaporins: novel functions and regulation properties. FEBS Lett 581, 2227–2236. 36 Gattolin S, Sorieul M & Frigerio L (2011) Mapping of tonoplast intrinsic proteins in maturing and germinating Arabidopsis seeds reveals dual localization of embryonic TIPs to the tonoplast and plasma membrane. Mol Plant 4, 180–189. 37 Gattolin S, Sorieul M, Hunter PR, Khonsari RH & Frigerio L (2009) In vivo imaging of the tonoplast intrinsic protein family in Arabidopsis roots. BMC Plant Biol 9, 133. 38 Paris N, Stanley CM, Jones RL & Rogers JC (1996) Plant cells contain two functionally distinct vacuolar compartments. Cell 85, 563–572. 39 Zheng H & Staehelin LA (2011) Protein storage vacuoles are transformed into lytic vacuoles in root meristematic cells of germinating seedlings by multiple, cell type-specific mechanisms. Plant Physiol 155, 2023–2035. 40 Swanson S & Jones R (1996) Gibberellic acid induces vacuolar acidification in barley aleurone. Plant Cell 8, 2211–2221. 41 Herman E & Larkins B (1999) Protein storage bodies and vacuoles. Plant Cell 11, 601–614. 42 Murphy KA, Kuhle RA, Fischer AM, Anterola AM & Grimes HD (2005) The functional status of paraveinal mesophyll vacuoles changes in response to altered metabolic conditions in soybean leaves. Funct Plant Biol 32, 335. 43 Hunter PR, Craddock CP, Di Benedetto S, Roberts LM & Frigerio L (2007) Fluorescent reporter proteins for the tonoplast and the vacuolar lumen identify a single vacuolar compartment in Arabidopsis cells. Plant Physiol 145, 1371–1382. 44 Di Sansebastiano GP, Paris N, Marc-Martin S & Neuhaus JM (2001) Regeneration of a lytic central vacuole and of neutral peripheral vacuoles can be visualized by green fluorescent proteins targeted to either type of vacuole. Plant Physiol 126, 78–86. 45 Martınez DE, Costa ML, Gomez FM, Otegui MS & Guiamet JJ (2008) ‘Senescence-associated vacuoles’ are involved in the degradation of chloroplast proteins in tobacco leaves. Plant J 56, 196–206. 46 Otegui MS, Noh Y-S, Martınez DE, Vila Petroff MG, Staehelin LA, Amasino RM & Guiamet JJ (2005) Senescence-associated vacuoles with intense proteolytic activity develop in leaves of Arabidopsis and soybean. Plant J 41, 831–844. 47 Bassham DC, Brandizzi F, Otegui MS & Sanderfoot AA (2008) The secretory system of Arabidopsis. Arabidopsis Book 65, 1. 988 48 Shan S-O, Schmid SL & Zhang X (2009) Signal recognition particle (SRP) and SRP receptor: a new paradigm for multistate regulatory GTPases. Biochemistry 48, 6696–6704. 49 Johnson AE & Van Waes MA (1999) The translocon: a dynamic gateway at the ER membrane. Annu Rev Cell Dev Biol 15, 799–842. 50 Singer SJ & Nicolson GL (1972) The fluid mosaic model of the structure of cell membranes. Science 175, 720–731. 51 Claessen JHL, Kundrat L & Ploegh HL (2012) Protein quality control in the ER: balancing the ubiquitin checkbook. Trends Cell Biol 22, 22–32. 52 Trombetta ES & Parodi AJ (2003) Quality control and protein polding in the secretory pathway. Annu Rev Cell Dev Biol 19, 649–676. 53 Liu J-X & Howell SH (2010) Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. Plant Cell 22, 2930–2942. 54 Kleizen B & Braakman I (2004) Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol 16, 343–349. 55 Ellgaard L & Helenius A (2003) Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4, 181–191. 56 Hebert DN & Molinari M (2007) In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol Rev 87, 1377–1408. 57 Pimpl P, Taylor JP, Snowden C, Hillmer S, Robinson DG & Denecke J (2006) Golgi-mediated vacuolar sorting of the endoplasmic reticulum chaperone BiP may play an active role in quality control within the secretory pathway. Plant Cell 18, 198–211. 58 Cai H, Yu S, Menon S, Cai Y, Lazarova D, Fu C, Reinisch K, Hay JC & Ferro-Novick S (2007) TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature 445, 941–944. 59 Pucadyil TJ & Schmid SL (2009) Conserved functions of membrane active GTPases in coated vesicle formation. Science 325, 1217–1220. 60 Nielsen E, Cheung AY & Ueda T (2008) The regulatory RAB and ARF GTPases for Vesicular Trafficking. Plant Physiol 147, 1516–1526. 61 Niemes S, Labs M, Scheuring D, Krueger F, Langhans M, Jesenofsky B, Robinson DG & Pimpl P (2010) Sorting of plant vacuolar proteins is initiated in the ER. Plant J 4, 601–614. 62 Wiederhold K & Fasshauer D (2009) Is assembly of the SNARE membrane fusion? J Biol Chem 284, 13143–13152. 63 Duman JG & Forte JG (2003) What is the role of SNARE proteins in membrane fusion? Plant Cell Physiol 285, 237–249. FEBS Journal 280 (2013) 979–993 ª 2012 The Authors Journal compilation ª 2012 FEBS L. Xiang et al. 64 Swanson SJ, Bethke PC & Jones RL (1998) Barley aleurone cells contain two types of vacuoles: Characterization of lytic organelles by use of fluorescent probes. Plant Cell 10, 685–698. 65 Matheson LA, Hanton SL & Brandizzi F (2006) Traffic between the plant endoplasmic reticulum and Golgi apparatus: to the Golgi and beyond. Curr Opin Plant Biol 9, 601–609. 66 Spang A (2009) On vesicle formation and tethering in the ER-Golgi shuttle. Curr Opin Cell Biol 21, 531–536. 67 Langhans M, Marcote MJ, Pimpl P, Virgili-L opez G, Robinson DG & Aniento F (2008) In vivo trafficking and localization of p24 proteins in plant cells. Traffic 9, 770–785. 68 Jensen D & Schekman R (2011) COPII-mediated vesicle formation at a glance. J Cell Sci 124, 1–4. 69 Yang YD, Elamawi R, Bubeck J, Pepperkok R, Ritzenthaler C & Robinson DG (2005) Dynamics of COPII vesicles and the Golgi apparatus in cultured Nicotiana tabacum BY-2 cells provides evidence for transient association of Golgi stacks with endoplasmic reticulum exit sites. Plant Cell 17, 1513–1531. 70 Russell C & Stagg S (2010) New insights into the structural mechanisms of the COPII coat. Traffic 11, 303–310. 71 Marti L, Fornaciari S, Renna L, Stefano G & Brandizzi F (2010) COPII-mediated traffic in plants. Trends Plant Sci 15, 522–528. 72 Pimpl P, Movafeghi A, Coughlan S, Denecke J, Hillmer S & Robinson D (2000) In situ localization and in vitro induction of plant COPI-coated vesicles. Plant Cell 12, 2219–2236. 73 Donohoe BS, Kang B-H & Staehelin L (2007) Identification and characterization of COPIa- and COPIb-type vesicle classes associated with plant and algal Golgi. Proc Natl Acad Sci USA 104, 163–168. 74 Mancias JD & Goldberg J (2005) Exiting the endoplasmic reticulum. Traffic 6, 278–285. 75 Neumann U, Brandizzi F & Hawes C (2003) Protein transport in plant cells: in and out of the Golgi. Ann Bot 92, 167–180. 76 Hanton SL, Renna L, Bortolotti LE, Chatre L, Stefano G & Brandizzi F (2005) Diacidic motifs influence the export of transmembrane proteins from the endoplasmic reticulum in plant cells. Plant Cell 17, 3081–3093. 77 Schoberer J, Vavra U, Stadlmann J, Hawes C, Mach L, Steinkellner H & Strasser R (2009) Arginine/lysine residues in the cytoplasmic tail promote ER export of plant glycosylation enzymes. Traffic 10, 101–115. 78 Hanton SL, Matheson LA, Chatre L & Brandizzi F (2009) Dynamic organization of COPII coat proteins at endoplasmic reticulum export sites in plant cells. Plant J 57, 963–974. Vacuolar protein sorting in plant cells 79 Lee HI, Gal S, Newman TC & Raikhel NV (1993) The Arabidopsis endoplasmic reticulum retention receptor functions in yeast. Proc Natl Acad Sci USA 90, 11433–11437. 80 Hwang I (2008) Sorting and anterograde trafficking at the Golgi apparatus. Plant Physiol 148, 673–683. 81 Hawes C, Osterrieder A, Hummel E & Sparkes I (2008) The plant ER – Golgi interface. Traffic 9, 1571–1580. 82 Connerly PL (2010) How do proteins move through the Golgi apparatus? Nat Educ 3, 60. 83 Cheng FY, Zamski E, Guo WW, Pharr DM & Williamson JD (2009) Salicylic acid stimulates secretion of the normally symplastic enzyme mannitol dehydrogenase (MTD): a possible defense against mannitol secreting fungal pathogens. Planta 230, 1093–1103. 84 T€ orm€akangas K, Hadlington JL, Pimpl P, Hillmer S, Brandizzi F, Teeri TH & Denecke J (2001) A vacuolar sorting domain may also influence the way in which proteins leave the endoplasmic reticulum. Plant Cell 13, 2021–2032. 85 Kirchhausen T (2000) Clathrin. Annu Rev Biochem 69, 699–727. 86 Traub L, Bannykh S, Rodel J & Aridor W (1996) AP2-containing clathrin coats assemble on mature lysosomes. J Cell Biol 135, 1801–1814. 87 Bryant N, Piper R, Weisman L & Stevens T (1998) Retrograde traffic out of the yeast vacuole to the TGN occurs via the prevacuolar/endosomal compartment. J Cell Biol 142, 651–663. 88 Fotin A, Cheng Y, Sliz P, Grigorieff N, Harrison SC, Kirchhausen T & Walz T (2004) Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature 432, 573–579. 89 Schmid SL (1997) Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem 66, 511–548. 90 McMahon HT & Boucrot E (2011) Molecular mechanism and physiological functions of clathrinmediated endocytosis. Nat Rev Mol Cell Biol 12, 517–533. 91 Hwang I & Robinson DG (2009) Transport vesicle formation in plant cells. Curr Opin Plant Biol 12, 660–669. 92 Bandmann V & Homann U (2012) Clathrinindependent endocytosis contributes to uptake of glucose into BY-2 protoplasts. Plant J 70, 578–584. 93 Gall L, Stan RC, Kress A, Hertel B, Thiel G & Meckel T (2010) Fluorescent detection of fluid phase endocytosis allows for in vivo estimation of endocytic vesicle sizes in plant cells with sub-diffraction accuracy. Traffic 11, 448–459. 94 Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D & Darnell J (2000) Molecular FEBS Journal 280 (2013) 979–993 ª 2012 The Authors Journal compilation ª 2012 FEBS 989 L. Xiang et al. Vacuolar protein sorting in plant cells 95 96 97 98 99 100 101 102 103 104 105 106 107 990 mechanisms of vesicular traffic. In Molecular Cell Biology. W.H. Freeman, New York, NY. Neuhaus J-M & Paris N (2005) Plant vacuoles: from biogenesis to function. Plant Cell Monogr 1, 63–82. Craddock CP, Hunter PR, Szakacs E, Hinz G, Robinson DG & Frigerio L (2008) Lack of a vacuolar sorting receptor leads to non-specific missorting of soluble vacuolar proteins in Arabidopsis seeds. Traffic 9, 408–416. Rojo E, Zouhar J, Carter C, Kovaleva V & Raikhel NV (2003) A unique mechanism for protein processing and degradation in Arabidopsis. Proc Natl Acad Sci USA 100, 7389–7394. Holwerda BC, Padgett HS & Rogers JC (1992) Proaleurain vacuolar targeting is mediated by short contiguous peptide interactions. Plant Cell 4, 307–318. Koide Y, Matsuoka K, Ohto M & Nakamura K (1999) The N-terminal propeptide and the C terminus of the precursor to 20-kilo-dalton potato tuber protein can function as different types of vacuolar sorting signals. Plant Cell Physiol 40, 1152–1159. Nakamura K & Matsuoka K (1993) Protein targeting to the vacuole in plant cells. Plant Physiol 101, 1–5. ~ez A, Sohn EJ, Robert S, Sanmartın M, Ord on Sanchez-Serrano JJ, Surpin MA, Raikhel NV & Rojo E (2007) Divergent functions of VTI12 and VTI11 in trafficking to storage and lytic vacuoles in Arabidopsis. Proc Natl Acad Sci USA 104, 3645–3650. Brown JC, Jolliffe NA, Frigerio L & Roberts LM (2003) Sequence-specific, Golgi-dependent vacuolar targeting of castor bean 2S albumin. Plant J 36, 711–719. Frigerio L, Jolliffe NA, Di Cola A, Felipe DH, Paris N, Neuhaus JM, Lord JM, Ceriotti A & Roberts LM (2001) The internal propeptide of the ricin precursor carries a sequence-specific determinant for vacuolar sorting. Plant Physiol 126, 167–175. Jolliffe NA, Brown JC, Neumann U, Vicre M, Bachi A, Hawes C, Ceriotti A, Roberts LM & Frigerio L (2004) Transport of ricin and 2S albumin precursors to the storage vacuoles of Ricinus communis endosperm involves the Golgi and VSR-like receptors. Plant J 39, 821–833. Koide Y, Hirano H, Matsuoka K & Nakamura K (1997) The N-terminal propeptide of the precursor to sporamin acts as a vacuole-targeting signal even at the C terminus of the mature part in tobacco cells. Plant Physiol 114, 863–870. Maruyama N, Mun LC, Tatsuhara M, Sawada M, Ishimoto M & Utsumi S (2006) Multiple vacuolar sorting determinants exist in soybean 11S globulin. Plant Cell 18, 1253–1273. Bednarek SY & Raikhel NV (1991) The barley lectin carboxyl-terminal propeptide is a vacuolar protein sorting determinant in plants. Plant Cell 3, 1195–1206. 108 Neuhaus JM, Sticher L, Meins F & Boller T (1991) A short C-terminal sequence is necessary and sufficient for the targeting of chitinases to the plant vacuole. Proc Natl Acad Sci USA 88, 100362–110366. 109 Schroeder MR, Borkhsenious ON, Matsuoka K, Nakamura K & Raikhel NV (1993) Colocalization of barley lectin and sporamin in vacuoles of transgenic tobacco plants. Plant Physiol 101, 451–458. 110 Nishizawa K, Maruyama N, Satoh R, Fuchikami Y, Higasa T & Utsumi S (2003) A C-terminal sequence of soybean b-conglycinin a-subunit acts as a vacuolar sorting determinant in seed cells. Plant J 34, 647–659. 111 Petruccelli S, Molina MI, Lareu FJ & Circosta A (2007) Two short sequences from amaranth 11S globulin are sufficient to target green fluorescent protein and beta-glucuronidase to vacuoles in Arabidopsis cells. Plant Physiol Biochem 45, 400–409. 112 Jolliffe NA, Craddock CP & Frigerio L (2005) Pathways for protein transport to seed storage vacuoles. Biochemistry 33, 1016–1018. 113 Vitale A & Hinz G (2005) Sorting of proteins to storage vacuoles: how many mechanisms? Trends Plant Sci 10, 316–323. 114 Nishizawa K, Maruyama N & Utsumi S (2006) The C-terminal region of a subunit of soybean b-conglycinin contains two types of vacuolar sorting determinants. Plant Mol Biol 62, 111–125. 115 Perez-Sala D, Boya P, Ramos I, Herrera M & Stamatakis K (2009) The C-terminal sequence of RhoB directs protein degradation through an endolysosomal pathway. PLoS One 4, e8117. 116 Behnke J, Schneppenheim J, Koch-Nolte F, Haag F, Saftig P & Schroder B (2011) Signal-peptide-peptidaselike 2a (SPPL2a) is targeted to lysosomes/late endosomes by a tyrosine motif in its C-terminal tail. FEBS Lett 585, 2951–2957. 117 Gough NR, Zweifel ME, Martinez-Augustin O, Aguilar RC, Bonifacino JS & Fambrough DM (1999) Utilization of the indirect lysosome targeting pathway by lysosome associated membrane proteins (LAMPs) is influenced largely by the C-terminal residue of their GYXXΦ targeting signals. J Cell Sci 112, 4257–4269. 118 Vitale A & Chrispeels M (1992) Sorting of proteins to vacuoles in plant cells. BioEssays 14, 151–160. 119 Hinz G, Menze A, Hohl I & Vaux D (1997) Isolation of prolegumin from developing pea seeds: Its binding to endomembranes and assembly into prolegumin hexamers in the protein storage vacuole. J Exp Bot 48, 139–149. 120 Kirsch T, Paris N, Butler JM, Beevers L & Rogers JC (1994) Purification and initial characterization of a potential plant vacuolar targeting receptor. Proc Natl Acad Sci USA 91, 3403–3407. 121 Seaman MNJ, Marcusson EG, Cereghino JL & Emr SD (1997) Endosome to Golgi retrieval of the FEBS Journal 280 (2013) 979–993 ª 2012 The Authors Journal compilation ª 2012 FEBS L. Xiang et al. 122 123 124 125 126 127 128 129 130 131 132 vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J Cell Biol 137, 79–92. Wang H, Rogers JC & Jiang L (2011) Plant RMR proteins: unique vacuolar sorting receptors that couple ligand sorting with membrane internalization. FEBS J 278, 59–68. Ahmed SU, Rojo E, Kovaleva V, Venkataraman S, Dombrowski JE, Matsuoka K & Raikhel NV (2000) The plant vacuolar sorting receptor AtELP is involved in transport of NH(2)-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana. J Cell Biol 149, 1335–1344. Happel N, Honing S, Neuhaus JM, Paris N, Robinson DG & Holstein SE (2004) Arabidopsis mu A-adaptin interacts with the tyrosine motif of the vacuolar sorting receptor VSR-PS1. Plant J 37, 678–693. Kalthoff C, Alves J, Urbanke C, Knorr R & Ungewickell EJ (2002) Unusual structural organization of the endocytic proteins AP180 and EPSIN 1. J Biol Chem 277, 8209–8216. Song J, Lee MH, Lee G-J, Yoo CM & Hwang I (2006) Arabidopsis EPSIN1 plays an important role in vacuolar trafficking of soluble cargo proteins in plant cells via interactions with clathrin, AP-1, VTI11, and VSR1. Plant Cell 18, 2258–2274. Oliviusson P, Heinzerling O, Hillmer S, Hinz G, Tse YC, Jiang L & Robinson DG (2006) Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis may interact with vacuolar sorting receptors. Plant Cell 18, 1239–1252. Miao Y, Yan PK, Kim H, Hwang I & Jiang L (2006) Localization of green fluorescent protein fusions with the seven Arabidopsis vacuolar sorting receptors to prevacuolar compartments in tobacco BY-2 cells. Plant Physiol 142, 945–962. da Silva LL, Taylor JP, Hadlington JL, Hanton SL, Snowden CJ, Fox SJ, Foresti O, Brandizzi F & Denecke J (2005) Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17, 132–148. Hara-Nishimura I, Shimada T, Hatano K, Yakeuchi Y & Nishimura M (1998) Transport of storage proteins to protein storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell 10, 825–836. Shimada T, Watanabe E, Tamura K, Hayashi Y, Nishimura M & Hara-Nishimura I (2002) A vacuolar sorting receptor PV72 on the membrane of vesicles that accumulate precursors of seed storage proteins (PAC vesicles). Plant Cell Physiol 43, 1086–1095. Molina MI, Otegui M & Petruccelli S (2006) Sunflower storage proteins are transported in dense vesicles that contain proteins homologous to the Vacuolar protein sorting in plant cells 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 FEBS Journal 280 (2013) 979–993 ª 2012 The Authors Journal compilation ª 2012 FEBS pumpkin vacuolar sorting receptor PV 72. J Biotechnol 9, 325–330. Shimada T, Fuji K, Tamura K, Kondo M, Nishimura M & Hara-Nishimura I (2003) Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proc Natl Acad Sci USA 100, 16095–16100. Jiang L & Rogers JC (2003) Sorting of lytic enzymes in the plant Golgi apparatus. Annu Plant Rev 9, 114–140. Park J, Oufattole M & Rogers J (2007) Golgimediated vacuolar sorting in plant cells: RMR proteins are sorting receptors for the protein aggregation/membrane internalization pathway. Plant Sci 172, 728–745. Mahon P & Bateman A (2000) The PA domain: a protease-associated domain. Protein Sci 9, 1930–1934. Hinz G, Colanesi S, Hillmer S, Rogers JC & Robinson DG (2007) Localization of vacuolar transport receptors and cargo proteins in the Golgi apparatus of developing Arabidopsis embryos. Traffic 8, 1452–1464. Park M, Lee D, Lee G-J & Hwang I (2005) AtRMR1 functions as a cargo receptor for protein trafficking to the protein storage vacuole. J Cell Biol 170, 757–767. Gillespie J, Rogers SW, Deery M, Dupree P & Rogers JC (2005) A unique family of proteins associated with internalized membranes in protein storage vacuoles of the Brassicaceae. Plant J 41, 429–441. Shen Y, Wang J, Ding Y, Lo SW, Gouzerh G, Neuhaus J-M & Jiang LW (2011) The rice RMR1 associates with a distinct prevacuolar compartment for the protein storage vacuole pathway. Mol Plant 4, 854–868. Hirst J, Barlow LD, Francisco GC, Sahlender DA, Seaman MNJ, Dacks JB & Robinson MS (2011) The fifth adaptor protein compex. PLoS Biol 9, e1001170. Boehm M & Bonifacino JS (2001) Adaptin: the final recount. Mol Biol Cell 12, 2907–2920. Dacks JB, Poon PP & Field MC (2008) Phylogeny of endocytic components yields insight into the process of nonendosymbiotic organelle evolution. Proc Natl Acad Sci USA 105, 588–593. Robinson MS & Bonifacino JS (2001) Adaptor-related proteins. Curr Opin Cell Biol 13, 444–453. Bonifacino JS & Traub LM (2003) Signals for sorting of transmembrane protein to endosomes and lysosomes. Annu Rev Biochem 72, 395–447. Sanderfoot AA, Ahmed SU, Marty-Mazars D, Rapoport I, Kirchhausen T, Marty F & Raikhel NV (1998) A putative vacuolar cargo receptor partially colocalizes with AtPEP12p on a prevacuolar compartment in Arabidopsis roots. Proc Natl Acad Sci USA 95, 9920–9925. Nakatsu F & Ohno H (2003) Adaptor protein complexes as the key regulators of Protein sorting in the post-Golgi network identification of AP complexes 991 L. Xiang et al. Vacuolar protein sorting in plant cells 148 149 150 151 152 153 154 155 156 157 158 992 recognition of sorting signals by AP complexes. Cell Struct Funct 429, 419–429. Owen DJ, Vallis Y, Pearse BM, McMahon HT & Evans PR (2000) The structure and function of the beta 2-adaptin appendage domain. EMBO J 19, 4216–4227. Slepnev VI & De Camili P (2000) Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat Rev Neurosci 1, 161–172. Stepp JD, Huang K & Lemmon SK (1997) The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J Cell Biol 139, 1761 –1774. Zwiewka M, Feraru E, M€ oller B, Hwang I, Feraru MI, Kleine-Vehn J, Weijers D & Friml J (2011) The AP-3 adaptor complex is required for vacuolar function in Arabidopsis. Cell Res 21, 1711–1722. Feraru E, Paciorek T, Feraru MI, Zwiewka M, De Groodt R, De Rycke R, Kleine-Vehn J & Friml J (2010) The AP-3 beta adaptin mediates the biogenesis and function of lytic vacuoles in Arabidopsis. Plant Cell 22, 2812–2824. Niihama M, Takemoto N, Hashiguchi Y, Tasaka M & Morita MT (2009) ZIP genes encode proteins involved in membrane trafficking of the TGN-PVC/ vacuoles. Plant Cell Physiol 50, 2057–2068. Burgos PV, Mardones GA, Rojas AL, daSilva LLP, Prabhu Y, Hurley JH & Bonifacino JS (2010) Sorting of the Alzheimer’s disease amyloid precursor protein mediated by the AP-4 complex. Dev Cell 18, 425–436. Abou Jamra R, Philippe O, Rass-Rothschild A, Eck SH, Graf E, Buchert R, Borck G, Ekici A, Brockschmidt FF, Nothen MM, Munnich A, Strom TM, Reis A & Colleaux L (2011) Adaptor protein complex 4 deficiency causes severe autosomal-recessive intellectual disability, progressive spastic paraplegia, shy character, and short stature. J Hum Genet 88, 788–795. Moreno-De-Luca A et al. (2011) Adaptor protein complex-4 (AP-4) deficiency causes a novel autosomal recessive cerebral palsy syndrome with microcephaly and intellectual disability. J Med Genet 48, 141–144. Janvier K, Kato Y, Boehm M, Rose JR, Martina JA, Kim B-Y, Venkatesan S & Bonifacino JS (2003) Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J Cell Biol 163, 1281–1290. Badolato R & Parolini S (2007) Novel insights from adaptor protein 3complex deficiency. J Allergy Clin Immunol 120, 735–741. 159 Robinson DG, Oliviusson P & Hinz G (2005) Protein sorting to the storage vacuoles of plants: a critical appraisal. Traffic 6, 615–625. 160 Chrispeels MJ (1983) The Golgi apparatus mediates the transport of phytohemagglutinin to the protein bodies in bean cotyledons. Planta 158, 140–151. 161 Hohl I, Robinson DG, Chrispeels MJ & Hinz G (1996) Transport of storage proteins to the vacuole is mediated by vesicles without a clathrin coat. J Cell Sci 109, 2539–2550. 162 Kim WT, Franceschi VR, Krishnan HB & Okita TW (1988) Formation of wheat protein bodies: involvement of the Golgi apparatus in gliadin transport. Planta 176, 173–183. 163 Hinz G, Hillmer S, Baumer M & Hohl I (1999) Vacuolar storage proteins and the putative vacuolar sorting receptor BP-80 exit the golgi apparatus of developing pea cotyledons in different transport vesicles. Plant Cell 11, 1509–1524. 164 Jiang L, Phillips TE, Hamm CA, Drozdowicz YM, Rea PA, Maeshima M, Rogers SW & Rogers JC (2001) The protein storage vacuole: a unique compound organelle. J Cell Biol 155, 991–1002. 165 Robinson DG, Hinz G & Holstein SEH (1998) The molecular characterization of transport vesicle. Plant Mol Biol 38, 49–76. 166 Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG & Jiang L (2004) Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16, 672–693. 167 Li L, Shimada T, Takahashi H, Ueda H, Fukao Y, Kondo M, Nishimura M & Hara-Nishimura I (2006) MAIGO2 is involved in exit of seed storage proteins from the endoplasmic reticulum in Arabidopsis thaliana. Plant Cell 18, 3535–3547. 168 Fuji K, Shimada T, Takahashi H, Tamura K, Koumoto Y, Utsumi S, Nishizawa K, Maruyama N & Hara-Nishimura I (2007) Arabidopsis vacuolar sorting mutants (green fluorescent seed) can be identified efficiently by secretion of vacuole-targeted green fluorescent protein in their seeds. Plant Cell 19, 597–609. 169 Wenzel D, Schauermann G, Von L€ upke A & Hinz G (2005) The cargo in vacuolar storage protein transport vesicles is stratified. Traffic 6, 45–55. 170 Hara-Nishimura I, Matsushima R, Shimada T & Nishimura M (2004) Diversity and formation of endoplasmic reticulum-derived compartments in plants. Are these compartments specific to plant cells? Plant Physiol 136, 3435–3439. 171 Toyooka K, Okamoto T & Minamikawa T (2000) Mass transport of proform of a KDEL-tailed cysteine proteinase (SH-EP) to protein storage vacuoles by endoplasmic reticulum-derived vesicle is involved in FEBS Journal 280 (2013) 979–993 ª 2012 The Authors Journal compilation ª 2012 FEBS L. Xiang et al. 172 173 174 175 176 177 178 179 180 181 182 183 protein mobilization in germinating seeds. J Cell Biology 148, 453–464. Mitsuhashia N, Hayashib Y, Koumotoa Y, Shimadaa T, Fukasawa-Akadac T, Nishimurab M & HaraNishimura I (2001) A novel membrane protein that is transported to protein storage vacuoles via precursoraccumulating vesicles. Plant Cell 13, 2361–2372. Mori T, Maruyama N, Nishizawa K, Higasa T, Yagasaki K, Ishimoto M & Utsumi S (2004) The composition of newly synthesized proteins in the endoplasmic reticulum determines the transport pathways of soybean seed storage proteins. Plant J 40, 238–249. Takahashi H, Saito Y, Kitagawa T, Morita S, Masumura T & Tanaka K (2005) A novel vesicle derived directly from endoplasmic reticulum is involved in the transport of vacuolar storage proteins in rice endosperm. Plant Cell Physiol 46, 245–249. Robinson DG, Hoh B, Hinz G & Jeong BK (1995) One vacuole or two vacuoles: do protein storage vacuoles arise de novo during pea cotyledon development? J Plant Physiol 145, 654–655. Van Der Wilden W, Herman EM & Chrispeels MJ (1980) Protein bodies of mung bean cotyledons as autophagic organelles. Proc Natl Acad Sci USA 77, 428–432. Shimada T, Nishimura M & Hara-Nishimura I (1994) Small GTP-binding proteins are associated with the vesicles targeting to vacuoles in developing pumpkin cotyledons. Plant Cell Physiol 35, 995–1001. Rouille Y, Rohn W & Hoflack B (2000) Targeting of lysosomal proteins. Semin Cell Dev Biol 11, 165–171. Ghosh P, Dahms NM & Kornfeld S (2003) Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol 4, 202–212. Doray B, Ghosh P, Griffith J, Geuze HJ & Kornfeld S (2002) Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science 297, 1700–1703. Deloche O, Yeung BG, Payne GS & Schekman R (2001) Vps10p transport from the trans-Golgi network to the endosome is mediated by clathrin-coated vesicles. Mol Biol Cell 12, 475–485. Costaguta G, Stefan CJ, Bensen ES, Emr SD & Payne GS (2001) Yeast GGA coat proteins function with clathrin in Golgi to endosome transport. Mol Biol Cell 12, 1885–1896. Matsuoka K & Neuhaus JM (1999) Cis-elements of protein transport to the plant vacuoles. J Exp Bot 50, 165–174. Vacuolar protein sorting in plant cells 184 Paris N & Neuhaus J-M (2002) BP-80 as a vacuolar sorting receptor. Plant Mol Biol 50, 903–914. 185 Raiborg C, Rusten TE & Stenmark H (2003) Protein sorting into multivesicular endosomes. Curr Opin Cell Biol 15, 446–455. 186 Maxfield FR & McGraw TE (2004) Endocytic recycling. Nat Rev Mol Cell Biol 5, 121–132. 187 Seaman MNJ (2005) Recycle your receptors with retromer. Trends Cell Biol 15, 68–75. 188 Reczek D, Schwake M, Schroder J, Hughes H, Blanz J, Jin X, Brondyk W, Van Patten S, Edmunds T & Saftig P (2007) LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of betaglucocerebrosidase. Cell 131, 770–783. 189 Iwaki T, Hosomi A, Tokudomi S, Kusunoki Y, Fujita Y, Giga-Hama Y, Tanaka N & Takegawa K (2006) Vacuolar protein sorting receptor in Schizosaccharomyces pombe. Microbiology 152, 1523–1532. 190 Wolfenstetter S, Wirsching P, Dotzauer D, Schneider S & Sauer N (2012) Routes to the tonoplast: the sorting of tonoplast transporters in Arabidopsis mesophyll protoplasts. Plant Cell 24, 215–232. 191 Braulke T & Bonifacino JS (2009) Sorting of lysosomal proteins. Biochim Biophys Acta 1793, 605–614. 192 Matsushima R, Hayashi Y, Yamada K, Shimada T, Nishimura M & Hara-Nishimura I (2003) The ER body, a novel endoplasmic reticulum-derived structure in Arabidopsis. Plant Cell Physiol 44, 661–666. 193 Herman EM (2008) Endoplasmic reticulum bodies: solving the insoluble. Curr Opin Plant Biol 11, 672–679. 194 Teckman JH & Perlmutter DH (2000) Retention of mutant a1-antitrypsin Z in endoplasmic reticulum is associated with an autophagic response. Am J Physiol Gastrointest Liver Physiol 279, 961–974. 195 Herman EM & Schmidt M (2004) Endoplasmic reticulum to vacuole trafficking of endoplasmic reticulum bodies provides an alternate pathway for protein transfer to the vacuole. Plant Physiol 136, 3440–3446. 196 Hayashi Y, Yamada K, Shimada T, Matsushima R, Nishizawa NK, Nishimura M & Hara-Nishimura I (2001) A proteinase-storing body that prepares for cell death or stresses in the epidermal cells of Arabidopsis. Plant Cell Physiol 42, 894–899. FEBS Journal 280 (2013) 979–993 ª 2012 The Authors Journal compilation ª 2012 FEBS 993