Chemistry 12 - Exam Review
advertisement

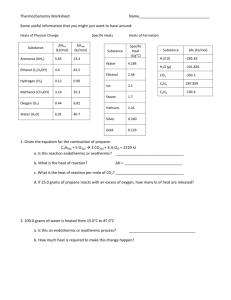
Chemistry 12 - Exam Review - Thermochemistry 1. 5.00 L of water at 70.0°C was cooled by adding 0.500 kg of ice at –10.0°C. Calculate the final temperature. 2. What would be the final temperature of 885 g water, initially at 25.0°C, if it were heated by the burning of 20.0 g of sulfur in excess oxygen to produce sulfur dioxide? 3. Calculate the enthalpy of reaction for: 2XO2(s) + CO(g) X2O3(s) + CO2(g) ∆H = –83.7 kJ XO2(s) + CO(g) XO(s) + CO2(g) ∆H = +25.1 kJ X3O4(s) + CO(g) 3XO(s) + CO2(g) ∆H = –50.2 kJ 3X2O3(s) + CO(g) 2X3O4(s) + CO2(g) 4. A 2.50 g sample of sucrose, C12H22O11(s) was burned in a 2.100 kg iron calorimeter immersed in 1.450 kg of water. The initial temperature of the system was 24.32°C and the final temperature was 30.20°C. Determine the heat of combustion of sucrose, under the conditions present inside the calorimeter. 5. 50.0 mL of 0.800 mol/L hydrobromic acid was added to 50.0 mL of 0.800 mol/L potassium hydroxide in a styrene cup. Initial temperature of both solutions was 23.18˚C. Final temperature was 26.38°C. Calculate the heat of reaction per mole of hydrobromic acid. 6. Any liquid stays at constant temperature while it is boiling. Why? 7. How much heat is released when 20.0 g of steam at 220.0˚C is cooled to ice at – 15.0˚C? 8. The average body temperature of a healthy human is ˚C. 37.0 What mass of steam at 140.0˚C must be added to 250.0 g of ice at –30.0˚C to produce water having the same temperature as the human body? 9. If 150.0 g of water at 0.0˚C is added to 100.0 g of water at 90.0˚C, what will be the final temperature of the water? 10. A swimming pool measures 10 m by 25 m by 3.5 m. Assuming the pool is full of water, how many kilojoules of heat energy are needed to raise the temperature of the water from 18.0˚C to 33.0˚C? ∆Hrx˚ = –2820 kJ 11. C6H12O6 + 6 O2 6 CO2 + 6 H2O Glucose is metabolized in the body according to the above reaction. How many grams of glucose must be burned to raise the temperature of the body from 34.0˚C to 37.0˚C for an 82.0 kg person? Assume that all the heat of combustion is used to heat the body. Assume also that the heat capacity per gram (specific heat) of the body is that of water. 12. Classify each process as exothermic or endothermic. a) discharging a battery c) photosynthesis b) burning alcohol d) baking a potato 13. Calculate ∆Hrxn˚ for the reactions below: a) 2 NaCl + H2SO4 Na2SO4 + 2 HCl(aq) (not balanced) b) C4H10 + O2 CO2 + H2O(g) c) Cu + 2 H2SO4(aq) CuSO4 + SO2 + 2 H2O(l) 14. Solar energy can preheat water for domestic hot water tanks. a) What quantity of heat is obtained from soar energy if 100.0 L of water is preheated from 10.0°C to 45.0°C? b) If natural gas costs 0.351¢/MJ, calculate the money saved if the volume of water in part (a) is heated 1500 times per year. 15. The solar-heated water in question 9 might be heated to the final temperature in a natural gas water heater. a) What quantity of heat flows into 100.0 L of water heated from 45.0°C to 70.0°C? b) At 0.351 ¢/MJ, what is the cost of heating 100.0 L of water 1500 times per year? 16. Under certain atmospheric conditions, the temperature of the surrounding air rises as a snowfall begins, because of the energy released to the atmosphere as water vapour changes to snow. What is the enthalpy change for the freezing of 1.00 t of moisture (water) in the air at 0.0°C to snow at 0.0°C? 17. On a winter day at –15.0ºC, a camper puts 750.0 g of snow into a pot over an open fire and heats the water to 37.0ºC. a) Assuming pure water and standard conditions, calculate the total energy needed to change the snow to water. b) Survival experts recommend that you do not eat snow even if you are stranded without water. Eating snow greatly increases the risk of hypothermia, the lowering of the body's core temperature. Use your calculations from part (a) to provide a scientific basis for this advice. 18. Canadian (CANDU) nuclear reactors produce energy by nuclear fission of uranium–235. If the molar enthalpy of fission for uranium–235 is 10 1.90 x 10 kJ/mol, how much energy can be obtained from the fission of 1.00 kg of uranium–235? 19. What mass of propane must be burned in order to heat 2.50 L of water at 10.0°C to steam at 120.0ºC? The enthalpy of formation of propane is –104.7 kJ/mol.