Introduction Particle Properties of Light
advertisement

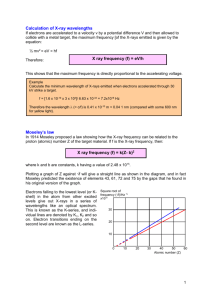
Introduction Until the early 20th century physicists used to explain the phenomena in the physical world around them using theories such a mechanics, electromagnetism, thermodynamics and statistical physics that are now known as classical theories. At the turn of the 19th century more and more experiments did show effects that could not be explained by these classical theories. This indicated a need for a new theory that we now know as quantum mechanics. Here we discuss a set of early experiments indicating the need for a new theory: particle properties of light • photoelectric effect, Compton effect, x-ray generation emission of light from atoms • discrete line spectra - energy levels, Franck-Hertz experiment wave properties of particles Particle Properties of Light • Until the beginning of the 1900’s all known properties of light (and in fact any type of electromagnetic radiation) were thought to only wave-like. • In the early years of the 20th century experimental observations did hint at the fact that light might also posses particle properties. I.e. that any radiation transports energy in quanta of energy that we now are used to call photons. • These observations were important in the early days of the developments of quantum mechanics. Now quantum mechanics is one of the best tested theories describing the physical world on the microscopic scale. • The knowledge of quantum mechanics is crucial for the understanding of basic physical phenomena on the microscopic (but increasingly also on the mesoscopic and macroscopic scale). • Here we discuss a number of phenomena and experiments in which the particle properties of light become apparent. Photoelectric Effect light of sufficiently high frequency (ultraviolet: λ ~ 1 – 400 nm) impinging on an Alkali metal surface leads to emission of electrons • Sodium (Na) Observation: • Electrons are emitted immediately (a few nanoseconds (10-9 s)) after light is switched on . Time for emission: try to explain it classically How to analyze effect in more detail? Measure the energy distribution of electrons. Photoelectric Effect light of sufficiently high frequency (ultraviolet: λ ~ 1 – 400 nm) impinging on an Alkali metal surface leads to emission of electrons • Sodium (Na) Observation: • Electrons are emitted immediately (a few nanoseconds (10-9 s)) after light is switched on . Photo Effect: Measurement of Photo Current • • dependence of stopping potential U0 on frequency of light dependence of stopping potential U0 on intensity of light Observations: • energy distribution is independent of intensity of light • intensity of light only changes the number of emitted electrons • maximum kinetic energy of measured electrons depends only of frequency of light Maximum Kinetic Energy of Photoelectron • maximum photo electron energy in dependence on frequency of light • • • threshold frequency for emission of electrons ν0 dependence of maximum kinetic energy of electrons is linear in frequency ν of light the proportionality constant is Planck’s constant h = 6.63 10-34 Js • the proportionality constant is independent of the metal used for the experiment Explanation of Photoelectric effect • note: Electrons can also be emitted from metal surface by heating up the metal, e.g. by running a large current through it. This process is called thermal emission. It is used, for example, in vacuum tubes for generating free electrons. In this case the heat (thermal energy) is providing the energy to extract the electron from the metal. The value of the work function can be determined this way as well and usually agrees well with the one that can be determined from the photoelectric effect. • exercise: Calculate the maximum kinetic energy of electrons emitted by the photo electric effect from a Potassium (Ka) metal surface with work function of 2.0 eV by ultraviolet photons with wavelength 350 nm. Nobel Prize in Physics (1906): Einstein "for his services to Theoretical Physics, and especially for his discovery of the law of the photoelectric effect" • Einstein’s explanation for the photoelectric effect: Light is composed of individual quanta of energy. We call those quanta photons. • The energy of a photon is given by • ν is the photon frequency • h is Planck’s constant Albert Einstein Germany and Switzerland Kaiser-Wilhelm-Institut (now MaxPlanck-Institut) für Physik Berlin, Germany b. 1879 (in Ulm, Germany) d. 1955 X-Rays • Generation of electromagnetic radiation by electrons impinging on a metal surface (inverse photo effect), i.e. conversion of kinetic energy of electrons to photons. Discovered by Wilhelm Rontgen (1895). schematic of an x-ray tube: • properties of X-rays: • propagates along straight lines (independent of magnetic or electric fields) • intensity increases with electron flux • faster electrons generate higher energy (more penetrating) X-rays • can expose photo sensitive materials • can generate phosphorescence or fluorescence light when interacting with hitting other materials X-Ray Spectra Tungsten (W): Molybdenum (Mo): observations: • continuous spectrum generated by decelerated charges (Bremsstrahlung) • lower cut-off wavelength λ (upper cut-off frequency ν) is set by maximum electron energy • cut-off is independent of material and only dependent on electron energy Minimum Wave Length of X-Rays lower cut-off wavelength λmin (upper cut-off frequency νmax) • • typical x-ray range of wave lengths: λ = 10-11 m to 10-8 m = 0.01 nm to 10 nm • exercise: Typical x-ray sources use electron energies of 50 keV. What is the corresponding minimum wave length or maximum frequency of emitted x-rays? (The first ever) Nobel Prize in Physics (1901) "in recognition of the extraordinary services he has rendered by the discovery of the remarkable rays subsequently named after him" Wilhelm Conrad Röntgen Germany Munich University Munich, Germany b. 1845 d. 1923 • The first x-ray image ever: The hand of Roentgen’s wife. X-Ray Diffraction • How to measure the wave length of x-rays? • Observe diffraction from a grating. For large diffraction angles to be observed period of diffraction grating needs to be comparable to wavelength of radiation (0.01 nm to 10 nm). • diffract x-rays in a solid state crystal in which atoms are spaced regularly on sub nanometer distances. e.g. a cubic crystal (NaCl) Bragg lattice planes: ai: lattice plane distances Point Scattering of X-Rays • scattering of an X-ray from a point-like scattering object (a single atom for example) • scattered waves from point-like sources interfere • the interference is constructive only in special directions • Which are these special directions? Bragg Diffraction first Bragg condition: • consider two paths I and II with same incidence and reflection angle θ: second Bragg condition • criterion on difference of lengths for paths I and II for constructive interference: Only if both conditions are fulfilled scattered x-ray light is observed in that direction. X-Ray Diffraction Measurement Apparatus • measure lattice plane distances with known wave lengths of x-rays • or measure x-ray wavelength with crystal with known lattice plane distances • exercise: Determine the lattice constant a of the NaCl cubic lattice from its density and molar mass. Nobel Prize in Physics (1915) "for their services in the analysis of crystal structure by means of X-rays" Sir William Henry Bragg William Lawrence Bragg 1/2 of the prize 1/2 of the prize United Kingdom United Kingdom London University London, United Kingdom Victoria University Manchester, United Kingdom b. 1862 d. 1942 b. 1890 (in Adelaide, Australia) d. 1971 Compton Effect • quantum theory of light states that a photon behaves as a relativistic particle with zero rest mass • consider an x-ray photon colliding with a single electron at rest • energy and momentum conservation can be used to analyze the problem Momentum of a Photon Photons in vacuum (like any electromagnetic wave) propagate with the velocity of light c ~ 3. 108 m/s. Thus they do behave like relativistic particles. energy of a relativistic particle: rest mass of photon: energy of a photon: resulting momentum of a photon: Compton Effect Calculation find the difference in wavelength λ’– λ between the incident and the scattered photon before after photon after collision electron - along direction of incidence - perpendicular solve for electron momentum: the total electron energy after the collision is: Compton Effect Calculation (4) with (0) results in: equating (3) and (5) we find: with the result is: Compton wave length kinetic energy rest energy Compton Effect Measurement Apparatus • The Compton effect can only be measured easily with x-rays because the Compton wavelength is small. Therefore the fractional change of the photon wavelength is smaller for larger wavelength. • Also the photons are scattered off weakly bound electrons in a metal rather than free electrons. Compton Effect Spectra λ λ’ • forward scattered spectrum shows only peak at input wave length λ • at scattering angles φ > 0 a Compton peak appears at the scattered wavelength λ’ • at φ > 0 a peak remains at the incident wave length due to scattering from more strongly bound electrons (looks like higher effective mass of electron m0* due to stronger binding to nucleus) • experiment to be demonstrated in class Nobel Prize in Physics (1927) "for his discovery of the effect named after him" "for his method of making the paths of electrically charged particles visible by condensation of vapour" • The observation of the Compton effect clearly demonstrates that photons carry a momentum and thus also confirms that light has particle properties. Arthur Holly Compton Charles Thomson Rees Wilson 1/2 of the prize 1/2 of the prize USA United Kingdom University of Chicago Chicago, IL, USA University of Cambridge Cambridge, United Kingdom b. 1892 d. 1962 b. 1869 (in Glencorse, Scotland) d. 1959