X-rays
advertisement

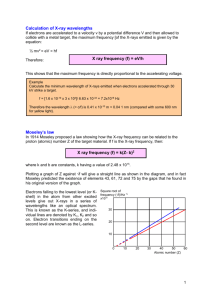
Ch6 X-ray • • • • • Producing X-ray Moseley formula X-ray diffraction Compton effect Absorption of x-rays Some Words • X-ray • Moseley’s model • Moseley's empirical formula • electromagnetic spectrum • Bremsstrahlung (轫致 辐射) • Characteristic x-ray • Bombard (轰击) • Graphite (石墨) • Vacancy • • • • • • • • • • Molybdenum (钼) Compton scattering Interaction Absorption coefficient Absorption edge Positron (正电子) Photoionization Pair formation Attenuate (衰减) Binding energy X-rays • X-rays mean electromagnetic radiation which has a wavelength shorter than that of ultraviolet light. 0.003-1nm, 1-500keV, or 1-100keV • X-rays can be produced when electrons, accelerated through a large potential difference, collide with a metal target. • A plot of x-ray intensity per unit wavelength versus the wavelength consists of sharp peaks or lines superimposed on a broad continuous spectrum (Bremsstrahlung). • The cut-off frequency of the Bremsstrahlung is determined by the KE of the impinging electrons. X-ray spectrum • X-ray is produced when a molybdenum target is bombarded with electrons of 35keV. • It includes Bremsstrahlung continuium & characteristic lines. hc 1.24 • There is cut-off nm 1eV V (kV ) frequency. min Brehmsstrahlung • "Bremsstrahlung" meaning "braking radiation" describes the radiation which is emitted when electrons are decelerated or "braked" when they are fired at a metal target. • Accelerated charges give off electromagnetic radiation, and when the energy of the bombarding electrons is high enough, that radiation is in the x-ray region of the electromagnetic spectrum. • It is characterized by a continuous distribution of radiation which becomes more intense and shifts toward lower wavelength when the energy of the bombarding electrons is increased. Characteristic x rays • There must be a vacancy in a subshell of an atom to produce characteristic x-rays. • The bombarding electrons can eject electrons from the inner shells of the atoms of the metal target. • Those vacancies will be quickly filled by electrons dropping down from higher levels, emitting x-rays with sharply defined frequencies associated with the difference between the atomic energy levels of the target atoms, called characteristic x-rays. K, L, M series in x-ray • K lines involve K shell of a metal atom. K (n 2 n 1), K (n 3 n 1), – K shell electron is knocked out of the atom. – An electron in a outer shell falls into the K shell. K (n 2 n 1), K (n 3 n 1), • L lines involve L shell of a metal atom. – L shell electron is knocked out of the atom. – An electron in a outer shell falls into the L shell. L (n 3 n 2), L (n 4 n 2), Moseley plot • When the square root of the frequencies of the characteristic xrays from the elements is plotted against the atomic number, a straight line is obtained. Moseley’s formula • Moseley showed that the characteristic x-rays followed a straight line when the atomic number Z versus the square root of frequency was plotted. • With the insights gained from the Bohr model, we can write his empirical (经验的) relationship as follows: A( z ) 2 ~K R 34 ( Z 1) 2 ~L R 365 ( Z 7.4) 2 X-ray diffraction • Like electron diffraction, x-rays can be diffracted by a crystal. • When x-rays are incident on a crystal in which atoms are arranged in regular array and act as an optical grating, the scattered waves will interfere in some directions. • The atoms in a crystal may be thought of as families of parallel planes known as Bragg planes. • The Bragg equation for maxima in the diffraction pattern is: n 2d sin Compton effect • Compton effect, Compton (1892-1962) showed that monochromatic X rays are scattered by graphite (石墨), and their wavelength increases by: • The collision between a photon and an electron is regarded as an elastic collision. • Compton wavelength of electron: h (1 cos ) m0 c 2h sin 2 m0 c 2 h m0c 2 h ' mc2 ' p p mv hc 0.00243nm 2 m0 c Compton scattering • Compton first to measure photon-electron scattering in 1922. • When the incoming photon gives part of its energy to the electron, then the scattered photon has lower energy. • The wavelength change in such scattering depends only upon the angle of scattering for a given target particle. Photon interaction with matter • There are three ways: – Photoionization, giving all of the energy to an electron. – Compton scattering: giving part of the energy to the electron and the remainder to a lower energy photon. – Pair production: At sufficiently high energies (>1.02MeV), the photon can create an electron positron pair. • Generally, x-ray’s interaction is mainly in first two ways. X-ray absorption • X-rays, like other electromagnetic radiation, are absorbed and scattered on passing through matter. • The transmitted intensity is given by: x x I I 0e intensity, I 0e – I0: incident – μ: absorption (Photoionization absorption + scattering) or attenuation coefficient, mass absorption coefficient, – x: the thickness of the material irradiated, • µ depends strongly on x-ray energy E and atomic number Z, and on the density ρand atomic mass A. Absorption Absorption edge Photon Energy • If the wavelength of the X-rays is reduced so that their energy is equal to one of the energies of the atomic levels of the absorber, a sudden increase in absorption is observed. • It corresponds to the photon energy to knock out an inner electron. • In X-ray spectrum, the K-absorption edge for each element is slightly less than that for the K-emission spectrum. • This feature of x-ray absorption edge has some applications, such as filter, spectrum differentiation & coronary angioplasty. Example1 • Question: The K absorption edge wavelength for uranium (Z=92) is 0.107Å and its Kα line is 0.126Å. Find the wavelength of its L absorption edge. • Answer: EK hc K n=inf 119.5keV () EL EK hc K 98.4keV E L 17.5keV () L BE L hcL hc 0.0709nm EL BE K L Kα K hc K Example 2 • Question: A beam of electrons with 100keV are bombarding the target. The binding energies for the inner shells for the target atom are listed in the table. Find the possible x-rays and their wavelength. shell K LI LII LIII MI MII MIII MIV MV electron 1s 2s 2p 2p 3s 3p 3p 3d 3d 2.520 0.505 0.410 0.393 0.230 0.227 BE (keV) 20.00 2.866 2.625 • Solution: Determine the energy level of subshells from the binding energy. Using selection rule to determine the possible transitions and then calculate the wavelengths. – Total: 12 lines: K-alpha (2), K-beta (2), L-alpha (7) The transitions M shell V IV Ⅲ Ⅱ Ⅰ 1 2 L shell 1 2 3 4 5 6 7 Ⅲ Ⅱ Ⅰ Lα 3d 3d 3p 3p 3s 2D 5/2 2D 3/2 2P 3/2 2P 1/2 2S 1/2 2p 2p 2s 2P 3/2 2P 1/2 2S 1/2 1s 2S 1/2 8 1 2 K shell Kα Kβ