Problem Set 3
advertisement

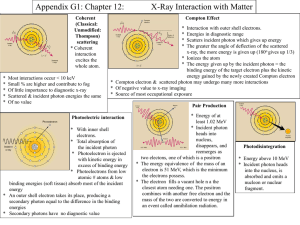
Problem Set 3 Due: See website for due dates Quantization of Light and Energy Reading: Taylor, Zafiratos, Dubson, Chapter 4; Tipler and Llewellyn, Chapter 3 Question A Explain physically how the introduction of Planck’s constant resolved the Ultraviolet Catastrophe (UVC)? Question B Explain why the existence of a cutoff frequency in the photoelectric effect more strongly favors a particle theory rather than a wave theory of light. Question C In our treatment of the photoelectric effect, we neglected conservation of momentum. Why is this approach justified? Suppose you take conservation of momentum into account – what qualitative change would it make to the energy of the electron that is emitted? Question D (i) In both the photoelectric effect and in the Compton Effect we have a photon colliding with an electron causing the electron to fly off. What then, is the difference between the two processes? (ii) Can Compton scattering occur with protons as well as electrons. For example, suppose a beam of X-rays is directed at a target of liquid hydrogen. Compared to Compton scattering with electrons, what similarities and differences would you expect? Problem 1 When light of wavelength 450 nm is shone on potassium, photoelectrons with stopping potential of 0.52 V are emitted. If the wavelength of the incident light is changed to 300 nm, the stopping potential is 1.90 eV. Using only these numbers together with the values of the speed of light and the electron charge, (a) find the work function of potassium and (b) compute a value for Planck’s constant. Answer: 2.24 eV; 6.63 1034 J.s Problem 2 The work function for tungsten is 4.6 eV. (a) If light is incident on tungsten, find the critical frequency, below which no electrons will be ejected, and the corresponding wavelength. Find the maximum kinetic energy of ejected electrons if tungsten is irradiated with light with (b) 200 nm and (c) 300 nm. Explain your answer to part (c). Answer: 1.1 1015 Hz, 270 nm, 1.6 eV, −0.5 eV. Problem 3 Millikan’s data for the photoelectric effect in lithium are shown in the table. (a) Graph these data and determine the work function for lithium. (b) Find a value of Planck’s constant from the graph in (a). (c) The work function for lead is 4.14 eV. Which of the wavelengths in the table would cause emission of photoelectrons from lead? Answer: 2.40 eV; 6.89 1034 J.s; 306 nm Incident (nm) 253.5 Vstop (V) 2.57 312.5 1.67 365.0 1.09 404.7 0.73 433.9 0.55 Problem 4 Using the Phet simulation of the Photoelectric Effect to describe in detail the (a) inability of the wave interpretation of light to correctly describe the data whereas the (b) correct description by the particle (or photon) interpretation of light. That is, describe for both models the following three plots Current vs. Battery Voltage Current vs. Light Intensity Electron energy vs. Light Frequency Website: http://phet.colorado.edu/en/simulation/photoelectric Problem 5 Use Compton’s equation to compute the value of for the figure. To what percent shift in the wavelength does this correspond when the incident wavelength is 0.0711 nm? Answer: 4.14 10 nm; 5.8% Problem 6 Compton used photons of wavelength of 0.0711 nm. (a) What is the energy of these photons? (b) What is the wavelength and (c) energy of the photons scattered at = 1800? (d) What is the recoil energy of the electrons? Answer: 1.747 104 eV; 0.0760 nm; 1.128 103 eV Problem 7 Consider a head-on, elastic collision between a massless photon (momentum p0 and energy E0 and a stationary free electron. (a) Assuming that the photon bounces directly back with momentum p and energy E, use conservation energy and momentum to find p. (b) Verify that your answer aggress with that given by Compton’s formula with = . Problem 8 (Challenge) Derive the Compton Shift equation ( 2 1 C (1 cos ) ).