Ch 11 – Phase diagram and alloy formation
advertisement

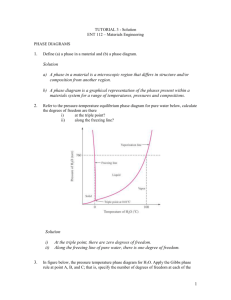
Ch 11 – Phase diagram and alloy formation... Ch 11 – Phase diagram and alloy formation 11.1-Introduction; 11.2-Alloy systems; 11.3-Total solid solubility; 11.4-Interpretation of phase diagram; 11.5-Solid solubility; 11.6-Phase diagram for total solid solubility; 11.7-Partial solid solubility; 11.10-Effect of alloy type on properties in metallic system; Text Book: V. B. John. Introduction to Engineering Materials. 4th Ed. Palgrave Macmillan; 2003 1 Ch 11 – Phase diagram and alloy formation... 11.1-Introduction: Let us start with some definitions and explanations of the terms which are going to be used throughout this chapter: Phase: a portion of a matter (one component or more) which is homogeneous (uniform), having same structure and same physical properties. It can be a solution, metal or chemical compound. For example; NaCl is a solid phase, water is a phase, a solution of salt dissolved in water gives a homogenous solution, termed as a single phase Phase diagrams (Equilibrium diagram): A graphical representation shows conditions at which distinct phases can occur at equilibrium. Thus it gives the amount of phases present in equilibrium w.r.t certain variables. One-component. Two -component (binary systems). Three-component (ternary system). 2 Ch 11 – Phase diagram and alloy formation... The Phase Rule: The phases represented in the phase diagram are related by the phase rule, Which states that: P + F = C + 2 (Gas, Liquid and solid) P = number of phases that can coexist in equilibrium; statistics C = number of components making up the phases; F = degrees of freedom, describes the minimum number of variables (Temperature, Pressure, Chemical Composition...etc.) that must be fixed in order to define a particular condition of the system.. Condensed systems have no gas phase and are insensitive to changes in pressure, so that one fewer variable needs to be specified, which results in the condensed phase rule: P + F = C + 1 (Liquid and solid only) Reading Materials... 3 Ch 11 – Phase diagram and alloy formation... P+F=C+2 P+F= 1+2 At a triple point (C), a condition in which three stability fields intersect. The phase rule (3 + F = 1 + 2) indicates that the variance is 0. Point C is therefore an invariant point; a change in either pressure or temperature results in the loss of one or more phases At point (a) a single solid phase exists 1 + F = 1 + 2, so F = 2. For point A (or any point in which only a single phase is stable), two degrees of freedom exist. Thus, the two variables (pressure and temperature) can be changed independently, and the same phase exist in equilibrium. a Reading Materials... 4 Ch 11 – Phase diagram and alloy formation... Condensed systems have no gas phase and are insensitive to changes in pressure, so that one fewer variable needs to be specified, which results in the condensed phase rule: P+F=C+1 P = number of phases that can coexist in equilibrium; C = number of components (alloying elements) that making up the phases; F = degrees of freedom (Temperature and Chemical Composition) At constant temperature and constant composition F = 0; P+0=C+1 For two component system C = 2 ... P = 3 For three component system C = 3 ...P = 4 The maximum number of phases can coexist in equilibrium is C (number of alloying compounds +1) 5 Ch 11 – Phase diagram and alloy formation... Phase diagram and cooling curves: Phase diagram can be constructed from information obtained from cooling curves which can be experimentally done. Several cooling curves for pure metals involved in the alloy construction as well as a mixed of different compositions cooling curves are used. Cooling curve for a pure metal Cooling curve for alloy system A cooling curve for each mixture is constructed and the initial and final phase change temperatures are determined. Then these temperatures are used for the construction of the phase diagrams. 6 Ch 11 – Phase diagram and alloy formation... 80% A 20% B (Volume fraction – Percent weight) Temperature-composition phase diagrams. 7 Ch 11 – Phase diagram and alloy formation... 8 Ch 11 – Phase diagram and alloy formation... Crystal growth: For pure metals; Slower rates of cooling will create larger crystals. Rapid cooling allows little time for element accumulation in the crystal, therefore, the crystals created will be smaller. As the rate of cooling increase, the number of nuclei (centre of crystallization) produced per unit volume increase. As a consequence of that, the number of crystals will increase...smaller size grains. The crystals of pure metal grow in a tree like crystal called “dendritic structure”. Many metals solidify from liquid in a dendritic manner...to form a skeleton type crystal. The outward growth ceases when the advancing dendrite arms meet an adjacent crystal. Then the dendrite arms thicken. 9 Ch 11 – Phase diagram and alloy formation... 11.2-Alloy systems. 10 Ch 11 – Phase diagram and alloy formation... 11.3-Total solid insolubility. Equilibrium diagram of binary alloys made up of two components which are soluble In the liquid state and insoluble in the solid state. Temperature-composition phase diagrams. 11 Ch 11 – Phase diagram and alloy formation... I Total solid insolubility. III Eutectic...point II IV Line AEB is called the liquidus curves; and Line CED as solidus line. I : Liquid from A+B. One phase (homogenous) II and III : Solid + liquid. Two phase II : Solid (A) + Liquid (A+B); Crystals of solid pure A , and liquid (A+B) enriched with B. III : Solid (B) + Liquid (A+B); Crystals of solid pure B , and liquid (A+B) enriched with A. IV : Solid A + solid B; Two phase. After solidifications we have an alloy of two metals, in which each metal kept its original structure. 12 Ch 11 – Phase diagram and alloy formation... Eutectic...point...mixture...alloy...reaction...temperature...etc. Eutectic mixture is a mixture at such proportions that the melting point is as low as possible. Furthermore all the constituents crystallize and separated simultaneously at this temperature from molten liquid solution. The cooling curve of such mixture is the same as the cooling curve of pure metal. Such a simultaneous crystallization of a eutectic mixture is known as a eutectic reaction. The temperature at which it takes place is the eutectic temperature, and the . composition and temperature at which it takes place is called the eutectic point. At point E: Solid (A) + Solid (B) + Liquid (A+B) co-exist at the same time. 13 Ch 11 – Phase diagram and alloy formation... L+A L+A+B The Phase Rule: P + F = C + 1 P+0=2+1 P = 3 (Three phases coexist in equilibrium) At the point (E), T is constant and composition is constant; F = 0 14 Ch 11 – Phase diagram and alloy formation... The primary crystal (A)could be “Dendritic” in form, and the remaining will be eutectic alloy (A + B) 15 Ch 11 – Phase diagram and alloy formation... 11.4-Interpretation of phase diagram. I think we interpret the phase diagram more than enough...for further reading refer to your text book... Section 11.4; Page 162. 16 Ch 11 – Phase diagram and alloy formation... 17 Ch 11 – Phase diagram and alloy formation... In the above solution the relative quantities were calculated? Percentage of (A) at point (U) = Uy/xy % Percentage of (L) at point (U) = Ux/xy % 18 Ch 11 – Phase diagram and alloy formation... 11.5-Solid solubility: Solid solution (SS): A two component system, form a single solid phase with one crystal structure. The atoms of both metals share the same crystal lattice...one component retained its structure after solidification and the other component give up its atoms. The component which retained its structure called “ Solvent”, and the one gives its atoms called “Solute”. 19 Ch 11 – Phase diagram and alloy formation... Restrictions to solid solution formation 20 Ch 11 – Phase diagram and alloy formation... Solid solutions can be classified as: • Complete solid solubility: At any conditions or concentration the two metals form a SS. An example Gold-Copper alloy; both have FCC; same melting temperature. • Limited solid solubility. Has a limited a mount of concentration. For complete solid solubility. The following factors must be satisfied: 1. The components must have the same crystal structures. i.e. FCC...FCC, and BCC...BCC...etc. 2. The size of the atoms and the lattice parameters must not vary by 7% for complete solid solubility. 3. The elements should belong to the same group in the periodic table; have the same electron configurations i.e. Same valency. For interstitial solid solubility. The conditions that must be satisfied: 1. The solute atoms must be small compared with the solvent atoms. Ex. (C), (H2), (N2)... 2. The inter atomic distance between the solvent atoms must be large.. 21 Ch 11 – Phase diagram and alloy formation... 11.6-Phase diagram for total solid solubility. Copper-Nickel phase diagram. An example of complete solubility in solid state: Both component have the same crystal structure FCC; similar valances; very close atomic radii and electro-negativities. Note that the solid solutions are commonly represented by lower case Greek letters. To find phase composition; for single phase point (A) has a composition as the overall composition of the alloy (40% Cu – 60% Ni). For two phase region see next slide: 22 Ch 11 – Phase diagram and alloy formation... Point (B) located in two phase region; first we draw a Tie line connecting the phase boundaries. CL, Co and C represent the composition. To find the phases amount in two phase region as mass fraction or percentage, we apply the lever principle “inverse lever rule”: At point B (alloy Cu%-Ni%) Amount of liquid with CL composition = S/(R+S) Amount of with C composition= R/(R+S) For calculation we use one component %, or we measure the distance. 23 Ch 11 – Phase diagram and alloy formation... 24 Ch 11 – Phase diagram and alloy formation... Coring: In-homogenous crystal structure called dendritic segregation, since the composition of the branches (dendrites) is varying from one arm to another. The centre of the crystal will be rich in Ni (high melting temperature element); the outer edges will be rich in copper. This structure is unwanted in fabrication processes, and leads to cracking and failure of the alloy during machining and manufacturing processes. So it must be eliminated. Heat and hold at annealing temperature for long time...How long is long? 25 Ch 11 – Phase diagram and alloy formation... 11.7-Partial solid solubility. 26 Ch 11 – Phase diagram and alloy formation... For an alloy with composition C1: Solidification will take place as complete solid solution. (a) The alloy is totally liquid, of composition C1 (b) Crossing the liquidus line, solid phase begins to form; with continued cooling more solid forms. (c) When solidification is complete, the structure will be one of cored (lead-rich) solid solution; with a uniform composition of C1. 27 Ch 11 – Phase diagram and alloy formation... For an alloy with composition C2: (d), (e) and (f) changes for the alloy with composition C2 will be similar to the changes for the alloy C1, discussed previously. (f) Just above the solvus line the micro structure consists of grains of composition C2. (g) Crossing the solvus line the solid solubility limit is exceeded, which results in the formation of small ? (tin-rich/not pure tin) phase particles rejected from solid solution as precipitate. may be precipitated either at the grain boundaries; or within the crystals; or at both sites. 28 Ch 11 – Phase diagram and alloy formation... 2 1 3 L L L L+ L+ L+ L++ +E +E At (s): The structure is composed of crystals of of composition q; and particles of composition r: 29 Ch 11 – Phase diagram and alloy formation... 30 Ch 11 – Phase diagram and alloy formation... 31 Ch 11 – Phase diagram and alloy formation... 32 Ch 11 – Phase diagram and alloy formation... 33 Ch 11 – Phase diagram and alloy formation... 34 Ch 11 – Phase diagram and alloy formation... 35 Ch 11 – Phase diagram and alloy formation... 36 Ch 11 – Phase diagram and alloy formation... 37 Ch 11 – Phase diagram and alloy formation... 38 Ch 11 – Phase diagram and alloy formation... 39 Ch 11 – Phase diagram and alloy formation... 11.10-Effect of alloy type on properties in metallic system. 40 Ch 11 – Phase diagram and alloy formation... 41 Ch 11 – Phase diagram and alloy formation... End of chapter-11 42 Ch 11 – Phase diagram and alloy formation... 11.9-Compound formation: It frequently occurs in phase diagrams of metals and ceramics that an A-B binary phase diagram will reveal one or more compounds formed from A and B. In that case, we divide the overall phase diagram into “panels”, each of which can be interpreted as a phase diagram between each pair of pure substances present in the system. () is a chemical compound of single phase (SS) with variable composition of limited solubility. 43 Ch 11 – Phase diagram and alloy formation... The number of “repeats” of the basic phase diagram tells how many such compounds there are. The “dividing lines” between the individual phase diagrams are intended to indicate compound formation, rather than an actual phase boundary. An example of compound forming in ceramic alloy system Four different compounds formed in this system. 44 Ch 11 – Phase diagram and alloy formation... Intermetallic compounds: Mg2 Sn Fe3C Fe C Fe3C 1538 3525 1148 BCC Cubic (diamond); Hexagon (graphite) very complex 45 Ch 11 – Phase diagram and alloy formation... 46 Ch 11 – Phase diagram and alloy formation... 47 Ch 11 – Phase diagram and alloy formation... 11.12-Ternary diagrams. 48 Ch 11 – Phase diagram and alloy formation... Note: In normal solidification rates, the rate is too fast for full equilibrium to be attained and the crystal will be “cored” 49