Trends periodic table lab
advertisement

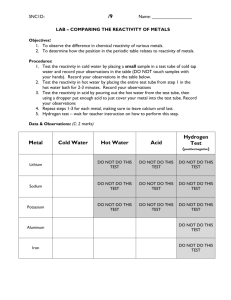
SCH 3UK UNIT 1 FORMAL LAB: PATTERNS IN THE PERIODIC TABLE THIS IS A THREE PART LAB TO BE COMPLETED AND FORMALLY PRESENTED IN A LABORATORY REPORT USING THE FORMAT SPECIFIED Overall Expectations A1. demonstrate scientific investigation skills (related to both inquiry and research) in the four areas of skills (initiating and planning, performing and recording, analysing and interpreting, and communicating); B2. investigate physical and chemical properties of elements and compounds B3. demonstrate an understanding of periodic trends in the periodic table EXPERIMENT 1 THE SEARCH FOR PATTERNS (THE REACTION OF METALS WITH WATER) In this lab we will react a number of active metals with water. From our observations we will attempt to establish the relationship between reactivity of an element and its position in the Periodic Table. NOTE: Before proceeding with this lab, read over the entire procedure very carefully and have your instructor ask you and your partner several oral questions. Have your data sheet initialed to indicate that this has been done. State the PROBLEM in question form. You also need to have a prepared and signed list of hazards associated with any chemicals you are using for the first time this semester.. CAUTION: FACE SHIELDS AND APRONS MUST BE WORN FOR THIS ACTIVITY! NEVER TOUCH ANY GROUP IA ELEMENT WITH BARE HANDS OR ALLOW CONTACT WITH WATER, EXCEPT AS DIRECTED. HYPOTHESES: Predict whether or not you think Li, Na and K will have similar reactions and properties since they are in family 1A. Also will reactivity increase or decrease from left to right? i.e. will Na or Mg be more reactive? Give reasons for your prediction based on past learning, experience, hint you received from the research of safety of these elements… PROCEDURE: 1. Half fill a clean 250 mL beaker with tap water and obtain a wire gauze with ceramic centre . Test the water with one piece each of red litmus, blue litmus and pH paper. Record your findings. (This is called a control.) 2. Using a square of paper towel (folded twice to four thicknesses ) obtain a piece of lithium form your instructor. Observe how it is cut so that you can describe its appearance and hardness. Record this on your data chart. 3. Gently dab the adhering oil from the metal using the corners of the paper towel. Stand at arm’s length away from the beaker. With the metal on the paper towel in one hand and the wire gauze directly above it in the other, carefully allow the metal to roll into the beaker of water. Immediately lower the gauze onto the beaker. When the reaction is complete test the resulting solution with one piece each of red litmus, blue litmus and pH paper. Record your findings. 4. Dispose of the reacted metal and water by flushing down the drain with plenty of water. Place the paper towel in the sink and completely wet it also. Then squeeze it out and place it in the metal can. Wash your hands with water and dry thoroughly before proceeding with the next step. 5. Repeat this procedure using each of the other metals listed on the data chart. NOTE: Ask your instructor to demonstrate the reaction of potassium with water. 6. You may have noticed an effervescent reaction in several of the above reactions, indicating a gas being produced. In order to investigate the nature of this gas, you will collect a small sample as follows. Fill the beaker nearly full of tap water. Fill an 18 mm X 150 mm test tube completely with water and cover the end with your thumb. Invert the test tube in the beaker and then remove your thumb while keeping the test tube upright and full of water. Pour out about one third of the water from the beaker. Drop a piece of calcium into the beaker and immediately place the mouth of the test tube over the calcium. Allow gas to bubble into the test tube until it is one third full of gas, then lift it quickly out of the beaker allowing the water to drain out. Set the test tube, mouth down on the desk. Now light a wooden splint. Lift the test tube up and tilt it on a 45° angle and insert the burning splint inside the mouth. Record the result. 1 SCH 3UK UNIT 1 FORMAL LAB: PATTERNS IN THE PERIODIC TABLE COMMON LABORATORY IDENTIFICATION TESTS Litmus test: Litmus paper Blue in Basic (or alkaline) solutions and reD in aciD pH paper Test: If the colour of the pH paper within 30 s of testing corresponds to a pH number less than 7, the solution is acidic, if greater than 7, basic, and if equal to 7, neutral. Burning splint test: If a colourless gas “pops” in the presence of a burning splint, the gas is probably HYDROGEN gas. Glowing splint test: If a glowing splint rekindles into a flame in the presence of a colourless, odourless gas, the gas is most likely OXYGEN. Lab #1- DATA SHEET NAME:___________________ LAB PARTNER ______________________ water ELEMENT lithium magnesium sodium calcium potassium SYMBOL FAMILY # DESCRIPTION OF ELEMENT (including coating) DESCRIPTION OF REACTION WITH WATER REACTION TIME (approx.) ORDER OF REACTIVITY 1= most/5= least LITMUS TEST red LITMUS TEST blue pH paper test colour/number SPLINT TEST 2 SCH 3UK UNIT 1 FORMAL LAB: PATTERNS IN THE PERIODIC TABLE EXPERIMENT 2 REACTION OF SELECTED ELEMENTS WITH DILUTE ACID (the search for patterns continues...) In this experiment you will investigate the reactivity of magnesium, silicon and aluminum (elements from families IIA, IIIA, and IVA) with dilute hydrochloric acid. Using your observations you will determine the order of reactivity of these elements. Your conclusion should support the generalization made in experiment #1. PRELAB: Before proceeding with this lab, read over the entire procedure carefully. Then write the PROBLEM and HYPOTHESIS. Also prepare a blank data table. If you are not already familiar with the hazards of any of the chemicals you will be using, read about these as well! PRECAUTIONS: FACE SHIELDS AND APRONS MUST BE WORN WHILE DOING THIS LAB!! PROBLEM: Write the problem to be investigated as a question. (HINT: your “problem” should be a single, coherent question incorporating the ideas in the first two sentences in the introductory paragraph above. An unacceptable example of the problem is “What is the reactivity of the elements magnesium, aluminum, and silicon?” as it is too simplistic. HYPOTHESIS: Predict the order of reactivity of the elements (most reactive to least reactive) being investigated in this lab. Support your prediction by citing evidence from the first lab. DO NOT START YOUR HYPOTHESIS WITH “I feel that ....” I think that ...” PROCEDURE 1. Into each of three clean test tubes (18 mm x 150 mm) measure out 5.0 mL of dilute (approx. 3 mol/L) hydrochloric acid. Place the test tubes in a test tube rack. 2. On small squares of paper towel obtain from your instructor small quantities of each of the following elements: aluminum, silicon, and magnesium. Record an accurate, concise description (colour, lustre form) of each on your data sheet. 3. Obtain two wooden splints and arrange for someone to set up and light a Bunsen burner in your area. There is no need for and in fact should not be a Bunsen burner for every lab partner pair. CAUTION: THE FLAME OF A BUNSEN BURNER IS ALMOST COLOURLESS-KEEP WELL BACK. Be prepared to light the splint on short notice. 4. Carefully drop the silicon into one of the test tubes of hydrochloric acid. Observe periodically over a 15 min. period and record whether any reaction occurs. If effervescence is observed, test the resulting gas by inserting a burning splint just inside the mouth of the test tube. 5. Repeat procedure 4 using aluminum instead of silicon. (HINT: if some effervescence is observed place a piece of masking tape over the mouth of the test tube for several minutes before testing with the splint.) 6. While allowing the silicon and aluminum to react, prepare a splint ready to light. Insert the magnesium into the 3rd test tube and observe. Does the test tube feel warmer as the reaction proceeds? Test with a burning splint just before the magnesium completely disappears. DISCARDING CHEMICALS: ALUMINUM: The reaction with aluminum may become quite vigorous after a while. To slow this reaction down, carefully pour the contents of the test tube into a small beaker half filled with water. Then place a piece of paper towel in the sink (not over the drain). Carefully pour the contents of the beaker onto the paper towel. Rinse the remaining aluminum residue in the test tube with water and pour out onto the paper towel. Allow the water to drain away. Then discard the wet paper towel with the aluminum residue into the large metal waste container. SILICON: Carefully pour ( without disturbing the solid) the acid from the test tube into the designated waste container in the fume hood. Add some water to the test tube to rinse the silicon. Pour off the water into the sink. Then dump the solid contents onto a piece of paper towel in the sink. Collect and return to the container designated by your teacher. MAGNESIUM: Into the waste container in the fume hood. CONCLUSION: Use paragraph style and complete sentence form. Include all of the following : -a brief summary of the reactions observed. (2-3 concise sentences) including the significance of the splint test -the order of reactivity of these elements with dilute hydrochloric acid (1 sentence) -was this order consistent with your prediction? -was your hypothesis upheld? -a general statement about the order of reactivity of elements in families IA through IVA -a general statement about the gaseous product of the reaction of metals with acids. 3 SCH 3UK UNIT 1 FORMAL LAB: PATTERNS IN THE PERIODIC TABLE SAMPLE DATA CHART (prepare a 1 page chart for recording your observations): ELEMENT silicon aluminum magnesium SYMBOL FAMILY # Description of element Description of reaction with hydrochloric acid Reaction time Splint test Order of reactivity EXPERIMENT 3: THE REACTION OF ELEMENTS WITH OXYGEN In this experiment you will investigate the reaction of a number of both metallic and non-metallic elements with oxygen. The results of these reactions should permit you to extend or modify some of the generalizations regarding reactivity trends you made in the previous two experiments. This is the main objective of the lab. A secondary result of this experiment is that it will allow you to learn something about the nature of acidic and basic solutions. PRELAB: Read over the entire procedure and begin your write-up by writing the PROBLEM (as a question please), the HYPOTHESIS and by preparing a blank, full page DATA TABLE. (see sample data table below) PROBLEM: In a concise, coherent question, phrase the problem (main objective) in this lab. HYPOTHESES: Predict whether the trend toward decreasing reactivity from left to right will be supported or not and cite scientific evidence to support your prediction (give your reason). Also, will the rend toward increasing reactivity down the family be supported or not? Cite scientific evidence. DATA: (make your own full page version of this chart) Keep it all on one page! Description Result of splint test Oxygen ELEMENT sodium phosphorus silicon sulfur carbon magnesium symbol/family Description of element metal or nonmetal Description of combustion reaction in AIR * Description of combustion reaction in OXYGEN Product description (before adding water!) Litmus test pH test Acidic/basic? nearly neutral? * Record intensity ( dull/bright/brilliant etc.) and colour of flame or glow ** Record colour, odour ( if obvious) and form (smoke/powder/lump) of product.3.4 4 SCH 3UK 1. 2. 3. 4. 5. 6. UNIT 1 FORMAL LAB: PATTERNS IN THE PERIODIC TABLE PROCEDURE Collect 2 bottles of oxygen from your teacher. Note that the bottles of oxygen are stored upright on the desk covered with a glass plate that may not form an “air-tight” seal. What does that suggest about the density of pure oxygen as compared to air? Prepare 2-3 deflagrating spoons (a.k.a. combustion spoons) as follows: scrape out any loose residue, heat to “red hot” in the tip of a well adjusted, hot Bunsen burner flame and hang over the edge of the sink to cool (about 3-5 min.) Describe the appearance and odour of oxygen (remove cover for a few second and smell). Test oxygen by inserting a glowing splint (light it , then blow it out) into the jar. Record. CAUTION: DO NOT PERMIT THE HOT DEFLAGRATING SPOON TO TOUCH THE GLASS JAR AT ANY TIME DURING THIS PROCEDURE!! Have your teacher place a pea-sized quantity of the element into one of the dry, cool, clean deflagrating spoons. Record the description of the element. Then heat the spoon in the hot tip of the burner flame. Note when/how it changes form as you are heating. When the burner flame acquires a white-yellow-orange tint (or other clue - see below) the element may be burning. Remove BRIEFLY from the flame to check for combustion. IF it continues to glow/flame in air, then the following sequence of steps should be carried out by one person only in quick succession: -note the colour/intensity of the combustion in air (have your partner record it.) -remove the glass plate from the mouth of a fresh bottle of oxygen. -insert the spoon into the bottle. Do not touch the sides! -slide the glass plate back over to cover the mouth as much as possible. -note the colour/intensity of the combustion oxygen. -allow reaction to go until flame/glow goes out. If the metal is not burning (no glow or flame) calmly return it to the burner and repeat the procedure. When the combustion is complete, with the lid still on, observe and record the appearance of the product (colourless gas/white smoke/white powder/ etc...) in the glass jar. Add tap water to the jar to a depth of about 1.0 cm. Remove the spoon, replace the glass plate with a stopper and shake vigorously for about ten seconds to dissolve as much of the product as possible. Perform the Litmus and pH tests on each solution. Record. PLEASE NOTE: SPECIAL INSTRUCTIONS for each of the elements listed on your data chart. sodium - some sodium my be left in the spoon when the reaction appears to be over. When adding water the reaction may BE VIOLENT. STAND AT ARM’S LENGTH!! phosphorus -Do this reaction in the fume hood! remove from burner flame when you see smoke and yellow-orange flame. silicon - heat until spoon is “red-hot” sulfur - Do this reaction in the fume hood! heat until sulfur melts to dark brown/black liquid, very hard to detect flame in air. carbon - heat until carbon has a dull red glow magnesium - CAUTION - DO NOT LOOK DIRECTLY AT BURNING MAGNESIUM ONCE IT IS IN THE GAS JAR!! Look out of the corner of your eye or take a “sweeping glance” the way you might look at the sun. It is OK in air. _____________________________________________________________________________________ _____________________________________________________________________________________ k;ln/ln 5 SCH 3UK UNIT 1 FORMAL LAB: PATTERNS IN THE PERIODIC TABLE For the following sections, include all answers in the proper section in your lab report CONCLUSIONS: Use sentence and paragraph format. In every conclusion you write you should address/discuss the following points (but do not use them as headings) -brief summary of the important observations Which elements burned in oxygen? Describe how they burned (flame/glow; intensity and colour; product). How did reactions in air compare with reactions in pure oxygen: (try to keep it to 2-3 sentences) -extent to which your hypothesis was upheld Describe the general reactivity pattern observed from left to right. ( Did reactivity appear to decrease or increase consistently from left to right or did the trend appear to change at some point?) Describe the general pattern observed down family IVB. (Did reactivity increase or decrease down family IVB?) How did these findings compare with your predictions? Was your hypothesis completely upheld, partially upheld, or totally incorrect according to the results of the lab? -sources of error or uncertainty Mention briefly some of the sources of error (unavoidable problems) encountered in this experiment (e.g. Are colour and intensity of flame alone reliable evidence on which to base reactivity trends?). -answer the question Answer the question you wrote under the heading “Problem” (hint: do not start with “the answer to the question is ...”) DISCUSSION: REGULARITIES AND TRENDS Refer to the results of all three labs when answering these questions. 1. Identify the liquid in which lithium, sodium and potassium are stored. Why is it necessary to store these elements in this liquid? 2. Which one of the elements sodium, silicon, or magnesium was the least reactive? Was this consistent with the pattern of reactivity observed in experiments 1 and 2? 3. Elements to the right of the “zig-zag” staircase line of the periodic table are classified as non-metals; those to the left are classified as metals. Classify silicon, phosphorus and sulfur. 4. Which of the three elements, silicon, sulfur or phosphorus was found to be least reactive? Do elements become more or less reactive moving to the right of the “zig-zag” line? 5. Compare silicon and carbon. Both are members of family IVB. Which was more reactive? 6. The pattern of reactivity for non-metals in family IVB is generally true for all non-metals. Does reactivity increase or decrease down non-metal families? 7. RECOPY and complete the following statements. Consult your experimental data as needed. Metal oxides when dissolved in water form _____________ (acidic/basic) solutions. Non-metal oxides when dissolved in water form _________(acidic/basic) solutions. 8. On a blank periodic table fill in (in pencil) the following details: - the line dividing metals and non-metals (label which is which) -the symbols of the elements investigated in this unit - the general reactivity tends discovered in this unit using arrows. the arrow begins at the least reactive element and the head points toward the most reactive element. Your chart should have a total of four arrows on it. 1 pointing down, 1 pointing up, 1 pointing to the left and 1 pointing to the right. 9. Which of the three elements from Family IA (sodium, lithium, potassium) was the most reactive? Which was the least reactive? 10. State the apparent order of reactivity as one proceeds down Family IA, i.e. does the reactivity increase or decrease down the family. 11. Which element from Family IIA, magnesium or calcium was more reactive? What is the apparent order of reactivity down Family IIA? 12. Compare adjacent Family IA and Family IIA elements investigated. Which is more reactive, magnesium or sodium? calcium or potassium? In general which family of elements is more reactive, Family IA or Family IIA? 13. Make a prediction about the apparent order of reactivity as one proceeds from left to right across the periodic table. 14. Predict several observations you might expect to make if you were to drop a piece of barium into a beaker of water. 15. Predict several observations you might expect to make if you were to drop a piece of cesium into a beaker of water. 16. What gas is produced when metals react with water? 17. What type of solution (acidic or basic) is formed when metals react with water? 6 SCH 3UK UNIT 1 FORMAL LAB: PATTERNS IN THE PERIODIC TABLE APPLICATION QUESTIONS 1. Predict 5 observations you would expect to make if you dropped a piece of rubidium metal into a beaker of water. 2. Predict what you would expect to see if you dropped a piece of beryllium into a beaker of water. 3. Which one of each of the following pairs of elements should be more reactive: a) iodine or chlorine b) selenium or sulfur c) rubidium or strontium d) francium or barium 4. An unknown element reacts with oxygen to form a colourless gas. This gas is then shaken with water to dissolve it. When the resulting solution is tested with pH paper a pH of 10 is recorded. Is this element a metal or a non-metal? Cite (give) experimental evidence to justify your answer. ASSESSMENT: Attached Rubric Please note all the components (parts of the lab report must be complete and included. A sample lab report file is also attached and an electronic copy is found in the class folder on the website. 7 SCH 3UK UNIT 1 FORMAL LAB: PATTERNS IN THE PERIODIC TABLE Grade 11 CHEMISTRY – Laboratory Report Assessment Rubric Kingston Collegiate & V.I. /50 OVERALL MARK: Section Specific Element Description for Level 4 What was done By what method What the main results were Conclusions drawn from these results Communication of information and ideas, in a scientific report format Communicates a summary of experiment information and results in a thoughtful, well-organized way. Statement of the purpose of the experiment Review of pertinent literature (including references) of the theory underlying the experiment and the procedure A hypothesis, or prediction, about the results Communication of information and ideas, in a scientific report format Reference the method used in laboratory manual/handout note any changes made Communication of information and ideas, in a scientific report format • Describes contents of paper. Name, date, etc.. Abstract (1 paragraph max.) • • • • • • • Method • Results/ Observations Data Tables • Titles • Column headings (including units) • A clear and accurate display of data Analysis Graphs • Appropriate axes and labels and units • A clear and accurate display of trends Calculations Complete and comprehensive calculations and units where appropriate include error Tables • Appropriate Title and labels with units • Calculated data Statement of results as evidence for or against hypothesis – draw on specific and representative results (use numbers) Explanation of results – with reference to data tables and graphs (use numbers you found to explain results argument) Revisit theory from introduction and connect with your results Discuss sources of error explain unusual results due to error experimental modifications to reduce error for next time Conclusion Application of your results to the real world Possible Future Studies References Within text – proper format (APA) Reference List – proper format (APA) Overall Impression Spelling and writing relatively error free Neat and complete and organized Logic and coherence of report as a whole Irrelevant theory deduction How you carried out the experiment: safety, cleanliness, safe use of laboratory equipment, technical skills in the lab • • • Discussion • - Experiment Execution Date:__________________________ Curriculum Expectations Title Introduction Name:_____________________________ Use of scientific terminology, including symbols, units, significant digits Use of various forms of communication, including diagrams, tables, graphs – with correct labels, headings, titles, etc. Understanding of concepts, principles, laws, and theories Knowledge of facts and terms Use of various forms of communication, including diagrams, tables, graphs – with correct labels, headings, titles, etc. Knowledge of facts and terms Understanding of relationships between concepts Proposing further courses of action, and/or making connections among science, technology, society, and the environment Communication of information and ideas, in a scientific report format Communication of information and ideas, in a scientific report format, including spelling and grammar Use of scientific terminology, including symbols, units, significant digits Skills and strategies of scientific inquiry: designing, planning, and performing experiments Technical skills in laboratory techniques Safe and correct: use of tools, equipment and materials; disposal of materials; cleanup Communicates all aspects of the introduction in a clear, thoughtful, wellorganized way. used proper scientific language and conventions Communicates all aspects of the method in a clear, thoughtful, well-organized way. used proper scientific language and conventions. IN TEXT CITATION OF THE METHOD IF PROVIDED AT THE END. Tables are used where appropriate and all units and titles are provided. Tables are introduced and described. Qualitative observations are included. Potential areas for ERROR are noted. Graphs and Tables are used where appropriate and included graph and axes titles and units, introduction before the graph in the text, and description in italics following the graph. Sample calculation including the general formula used are shown and the exact data used in the sample is explained… “data to show the sample calculation is used from trial one on observation table 2” Statement of the results is clear and refers to the hypothesis. Correct scientific terms are used to communicate results and EXPLAIN the results. Error (systematic and Random error) and the effects that the error would have on the results is discussed. Next steps and applications to society of the results or concepts examined in the lab are discussed with sources where appropriate. References are made IN TEXT and include a works cited page Communication is almost completely free of error in spelling and grammar. It is completely free of error of scientific terminology, symbols, units and significant digits Experiment was designed appropriately where applicable to assessment. Lab was carried out free from safety issues, reminders from teachers and the lab station was properly set up and put away to leave a clean work area. Materials were always treated properly and disposed of correctly. 8