Atomic Emission Spectra
advertisement

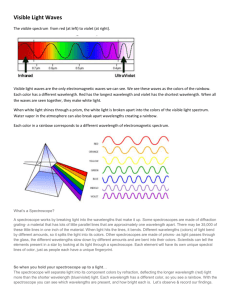
EXPERIMENT Atomic Emission Spectra 30 Prepared by Zach Gray and Edward L. Brown, Lee University Students will observe the emission spectra of various atoms both visually and with a direct reading spectroscope. The wavelengths obtained from the spectroscope will be used to calculate the energies of Hydrogen’s electronic transitions. Students will use a colorimeter to generate a standard curve for blue food coloring. A P P A R A T U S Direct Reading Spectroscope OBJECTIVE APPARATUS AND CHEMICALS Spectrum Tube Power Supply (Caution! High Voltage) Gas Discharge Tubes (Hydrogen, Helium, Neon, Mercury) Incandescent bulb Fluorescent bulbs (white, red, green, blue and black) Laser (red) MeasureNet Colorimeter C H E M I C A L S Ca(NO3)2 (s), KNO3 (s), Ba(NO3)2 (s), NaNO3 (s) (flame tests) Food Coloring (Blue and Yellow) Introduction Electrons in atoms normally occupy the lowest energy states possible. Such an atom is said to be in the ground state. However, electrons can be excited to high energy states when they absorb energy. This energy can be provided by heat, light, or an electrical discharge. The excited electrons will then return to lower energy states, eventually returning all the way to the ground state. As the excited electrons return to lower energy states, they must release their excess energy. Often, this released energy appears in the form of light, with each atom or molecule releasing a single photon of light for each electron energy transition it makes. In the case of Hydrogen, a single electron can be excited from the first energy level (n = 1) to a higher energy level (n = 2, 3, 4, 5, 6, or 7). The path this electron uses to return to the ground state (n = 1) can involve many individual electronic transitions each producing electromagnetic radiation (a photon) with a specific amount of energy. This energy can be calculated once the specific n Copyright 2005 Chem21 LLC. No part of this work may be reproduced, transcribed, or used in any form by any means – graphic, electronic, or mechanical, including, but not limited to, photocopying, recording, taping, Web distribution, or information storage or retrieval systems – without the prior written permission of the publisher. For permission to use material from this work, contact us at info@Chem21Labs.com. Printed in United States of America. values of the transition are known. Several different chemists (Lyman, Balmer, Paschen, Brackett) using different instruments to observe UltraViolet (UV), Visible, and InfraRed (IR) waves, reported the “series” of electromagnetic radiations (see Figure 1) coming from excited Hydrogen atoms. Note that the Lyman, Balmer, Paschen, and Brackett series are composed of transitions that end at n = 1, 2, 3 and 4, respectively. A combination of all of an element’s “series” gives its emission spectrum – an electronic “fingerprint” of the element. An element has a UV fingerprint, a Visible light fingerprint, an IR fingerprint, etc. In today’s lab you will observe the Visible light fingerprint of Hydrogen, Neon, and Mercury. Figure 1 Hydrogen Emission Spectrum Figure 2 Neon Emission Spectrum Figure 3 Mercury Emission Spectrum Figure 4 An element’s emission spectrum is also called a “line spectrum” – note the “lines” in Figures 2 – 4. Each line corresponds to a specific transition of an excited electron to a lower energy state. Visible light can be separated into the colors of the rainbow (ROYGBIV) by a prism. However, the colors should be written as VIBGYOR, since electromagnetic scales have the highest energy radiation on the left – just as UltraViolet radiation has more energy than InfraRed, Violet light has more energy than Red light. The color of visible light emitted by a particular atom depends Experiment 30 30-2 upon how much energy the electron releases as it transitions to a lower energy level. The energy levels that are allowed for each atom depend upon the number and arrangement of protons and electrons in the atom. Since each element has different energy states available to it, the photons, and the associated colors that are released when an electron falls to a lower energy state is unique to that element. This set of individual colors emitted by an element is called its emission (also atomic) spectrum. The wavelength (λ) of the emitted radiation determines the type of radiation. A wavelength is the distance between identical points in adjacent cycles of a wave (λ in Figure 5). λ Figure 5 The three types of cone cells (yellow, green and blue) in the human eye establish the limits of the visible region as light with wavelengths between 400 nm and 700 nm (Figure 6). Figure 6 The color of light is related to its wavelength, which is related to its frequency (ν) and the energy of its photons (E) as shown in Equation 1. λv = c and E = hv Equation 1 where “c” is the speed of light in a vacuum (3.0 x 108 m/sec) and “h” is Planck’s constant (6.626 x 10-34 J-sec). These two relationships can be combined to give a third relationship: E= Experiment 30 hc Equation 2 30-3 Note that shorter wavelengths of light correspond to higher frequencies and higher photon energies. Once the wavelength of an atomic emission is known, the energy of that emission can be calculated from Equation 2. To measure the various wavelengths emitted by a particular element, they must first be separated. Because the human eye cannot separate the colors striking the cones, the electrical impulses from the blue, green and yellow cones are “mixed” and the brain interprets the impulse as a single color. However, if the light is first passed through a prism or diffraction grating, the individual wavelengths can be separated. The direct reading spectroscope contains a diffraction grating and a scale where spectra similar to Figures 1 – 3 are seen and separated into its various wavelengths, an effect very similar to the way the sun’s white light is separated into its component colors by rain droplets. The Hydrogen Spectrum The Hydrogen atom has one electron that is in the 1s sublevel in its ground state. This electron can be excited to a higher energy level where n = 2, 3, 4, 5, 6, 7, etc (see Figure 1). In fact, passing electricity through a tube of Hydrogen gas will excite millions of Hydrogen atoms so that there are millions of electrons at the 2nd – 7th energy levels. These excited electrons begin to “fall” to lower energy levels and emit photons at particular wavelengths. However, most of these photons possess wavelengths that our eyes cannot detect (either IR or UV). The numbers shown in Figure 1 show only four electronic transitions that emit photons with wavelengths between 400 nm and 700 nm (the visible portion of the spectrum). These four transitions are part of the Balmer series where the transition is from a higher n level to the n=2 level. For atoms that contain only one electron (i.e. the Hydrogen atom), the theory of atomic structure proposed by Niels Bohr can be used to calculate wavelengths for transitions between particular electronic energy levels of the atom. The equation relating energy levels in the hydrogen atom and the wavelengths of the spectral lines was originally fitted empirically to experimental data and has the form: 1 1 1 RH 2 - 2 = nL nH λ Equation 3 Where R is the Rydberg constant (1.0968 x 107 m-1), and nL and nH are the principal quantum numbers that correspond to the beginning and ending energy levels of the electron’s transition. For the purposes of this experiment, Equation 3 is rearranged to give: 1 1 9.12×10-8m = n L2 n H2 λ Equation 4 Substituting the wavelength of the emitted photon into Equation 4 gives a number that is equal to the left-hand side of Equation 4. From this number it is possible to calculate the exact energy levels (nL and nH) that were involved in the electronic transition that gave rise to a particular spectral line. For example, if 1/ nL2 - 1/ nH2 is found to be 0.75, then nL must be 1 and nH must be 2 as shown: 1 1 1 1 3 - 2 = - = = 0.75 2 1 2 1 4 4 Experiment 30 Equation 5 30-4 The spectral line in question represents the energy emitted when the electron falls from the second quantum level to the first. Any electron falling to the first level from any higher energy state (e.g. nH = ∞) will have values between 0.75 and 1 since 1 1 1 1 - 2 = - 1 2 nL nH 1 Equation 6 When an electron falls from the third quantum level, nH = 3, to the second, nL = 2, the calculation gives: 1 1 1 1 - 2 = - = 0.139 2 2 3 4 9 Equation 7 For any electron falling to the second level from any higher energy state (e.g. nH = ∞), the calculation of will have values between 0.250 and 0.139. Sample Calculation: One of the spectral lines for hydrogen occurs at a wavelength of 435 nm. Calculate the electronic transition responsible for this spectral line. Substitute the value for λ in Equation 4. 1 1 9.12 × 10-8 m = = 0.2096 n L2 n H2 4.35 × 10-7 m Since 1 1 - 2 2 nL nH is less than 0.250 and greater than 0.139 (see above), the electron falls to the second level (nL = 2) from a higher energy level. Substitute 2 for nL and solve for nH. 1 1 = 0.2096 22 n H 2 n H 2 24.7 nH 5 Therefore, the electronic transition corresponding to the 435 nm spectral line for hydrogen is 5 → 2. Viewing Other Light Sources With The Spectroscope As described earlier each element emits a unique line spectrum when its electrons are excited by heat or electricity. Therefore, line spectra can be used to determine the elemental composition of an object. Helium was discovered when a spectroscope was used to view the sun. No known atom at the time had a line spectrum like the one observed so the scientists knew it must be a new element. The element was named after the Greek word for sun Helios. In today’s lab, we Experiment 30 30-5 will “discover” the element in fluorescent lights by comparing the fluorescent light spectrum to the spectra of Hydrogen, Neon and Mercury. An incandescent light emits all the colors of the rainbow and humans perceive its light as white. Several colored lights (red, blue, green black) will be viewed through the spectroscope and large sections of the visible region will be missing. For example the red light bulb you will observe in this lab emits all the colors of the rainbow, but the material that coats the glass absorbs most of the “non-red” radiation. Flame Tests When heated in a flame, electrons in atoms will occupy excited states. When these electrons transition to a lower energy state, photons of light possessing specific wavelengths (and having specific colors) are emitted. This emission spectrum gives the atom a unique flame color. By heating the solid on a porcelain spatula, it is possible to produce a color that corresponds to that element’s line spectrum. Colorimeter A colorimeter measures the amount of light absorbed by a solution when light of a particular wavelength is shone through it. A colorimeter is typically used to measure the concentration of a known solute in a solution by comparing the solute’s absorbance to a graph that shows the solute’s absorbance at several different concentrations. This is called a standard curve. The points that make up the standard curve should yield a straight line (y = mx + b) and the slope (m) and intercept (b) of that line provide a mathematical relationship between absorbance and concentration. In this lab, a colorimeter will shine red light through the five solutions that contain varying amounts of blue food coloring. Although the blue coloring will be mixed with yellow coloring to produce different shades of green, it is the blue coloring that will absorb the red light. Recall that when something appears blue, it contains a molecule that absorbs the complementary color of blue (which is red/orange). Then the object reflects back to us all of the colors of the rainbow (ROYGBIV) without the red/orange ( _ _ YGBIV). When our eyes are exposed to “YGBIV” light, we see blue. Also note that solutions that appear yellow absorb very little red light since yellow and red are close to each other in the visible spectrum. Each of the five solutions will absorb the red light to a different extent that correlates with the amount of blue food coloring in the solution. A graph of the Absorbance vs. concentration of blue dye provides a standard curve that can be used to determine the concentration of your Experiment 30 30-6 unknown. Simply place the unknown solution in the colorimeter and record its Absorbance. The absorbance reading can be substituted for “y” in the equation (y = mx + b) of the standard curve line. Simple mathematical manipulations provide the value of “x” (the concentration of the blue food coloring in the unknown solution). Safety Exercise extreme caution with the high voltage power supply, as severe shocks are possible! Do not touch the front of the power supply while it is plugged in. Do not touch or attempt to remove the discharge tubes from the high voltage power supply. In addition to the risk of electrical shock, the tubes become very hot with use. The power supplies must be turned off and unplugged before changing discharge tubes. Do not play around with laser (i.e. point in eyes) - only view the reflection of the light the laser makes on the white piece of paper. Work in Pairs PROCEDURE A. Hydrogen Discharge Tube 1. Aim the direct reading spectroscope at the hydrogen discharge tube - your Instructor can help you correctly position the spectroscope. 2. Look through the eyepiece of the spectroscope. To the right, you will see a wavelength guide (400 nm – 700 nm) and three lines of different colors. Record the color and wavelength of each of the three lines [Data Sheet]. There is a fourth line, but it is very difficult to observe. The wavelength and color of this line is given in the Data Sheet). 3. Use Equations 4 – 7 to determine the specific energy levels involved in the transitions observed for each of the four wavelengths recorded [Online Lab Sheet]. B. Helium, Neon, and Mercury Discharge Tubes 4. Aim the direct reading spectroscope at each of the remaining discharge tubes (Helium, Neon, and Mercury). Record the color and wavelength of the brightest four lines [Data Sheet] C. Identification of the Element in Fluorescent Lights 5. Aim the direct reading spectroscope at the fluorescent light bulb set out by your professor (it is the first bulb on the light fixture with six lights). Record the color and wavelength of the brightest four lines [Data Sheet]. Compare the fluorescent bulb’s line spectrum to the line spectrums produced by the hydrogen, neon, and mercury discharge tubes. Identify the element used inside fluorescent bulbs [Data Sheet]. D. Colored Lights and Lasers 6. Aim the direct reading spectroscope at one of the colored light bulbs set out by your professor. Describe the spectrum produced by the light [Data Sheet]. 7. Repeat for the other colored bulbs [Data Sheet]. Experiment 30 30-7 8. Point a laser pointer at a plane white sheet of paper, point the direct reading spectroscope at the dot generated by the laser (Do Not Point The Laser At The Spectroscope!!). 9. Describe the spectrum produced by the laser [Data Sheet]. E. Flame Tests 10. Place calcium nitrate (solid) on a porcelain spatula. 11. Hold the spatula over the flame. 12. Record the color of the flame [Data Sheet]. 13. Repeat for the remaining solids and record the flame colors [Data Sheet]. F. Colorimeter 14. The MeasureNet workstation should be ON and a colorimeter attached to it. Maaiinn M 15. Press [M Meennuu]. F66] (Colorimetry/Flour./Turb./Lum.). 16. Press [F F11] (Colorimetry). 17. Press [F F11] (Red Light). 18. Press [F F11] (Kinetics). 19. Press [F 20. At the calibration screen place a cuvette filled with DI water into each slot (labeled R and S) of the colorimeter. Do Not Force the cuvette! If it doesn’t fit easily, rotate it 90º. If it still doesn’t fit, contact your Instructor. Enntteerr] 21. Press [E 22. Wait for colorimeter to calibrate. Diissppllaayy]. 23. Click [D 24. Obtain one of the cuvettes with a known concentration of blue food dye (labeled 1-5) from the instructor bench. 25. Place the cuvette in the slot labeled S (leave the cuvette filled with DI water in the R slot for the entire lab). Record the absorbance [Data Sheet] next to its Solution Number. 26. Repeat Steps 24 - 25 for the remaining four cuvettes containing solutions of blue food dye (labeled 1-5). 27. Follow online instructions to create your standard curve [Online Lab Sheet]. G. Determining the Concentration of an Unknown 28. Obtain one of the unknown cuvettes. Record the Unknown number [Data Sheet]. 29. Place the cuvette in the slot labeled S and record the absorbance [Data Sheet]. 30. Use the slope and y-intercept of your standard curve to determine the % blue dye in your unknown [Online Lab Sheet]. Experiment 30 30-8 Laboratory 30 Student Data Sheet A. Hydrogen Discharge Tube 1 2 3 4 B. Wavelength (λ) (nm) 410 Color of Spectral line violet Helium, Neon, and Mercury Discharge Tubes Helium Color of Spectral line Wavelength, λ (nm) 1 2 3 4 Mercury Neon Color of Spectral line C. Identification of the Element in Fluorescent Lights What element is inside fluorescent lights? Hydrogen || Helium || Neon || Mercury (Circle One) Colored Lights and Lasers Incandescent – Green – Black – Red – Blue – Red Laser – Experiment 30 Wavelength, λ (nm) 1 2 3 4 1 2 3 4 D. Color of Spectral line Wavelength, λ (nm) Fluorescent Light Color of Spectral line Wavelength (λ) (nm) 1 2 3 4 30-9 E. F. G. Flame Tests Calcium Nitrate – Barium Nitrate – Sodium Nitrate – Potassium Nitrate – Colorimeter Solution Blue : Yellow Ratio 1 1:1 2 2:1 3 3:1 4 4:1 5 5:1 Absorbance Determining the Concentration of an Unknown Unknown Number Unknown’s Absorbance Experiment 30 30-10 Name: Partner’s Name: Laboratory 30 Instructor Data Sheet A. Hydrogen Discharge Tube 1 2 3 4 B. Wavelength (λ) (nm) 410 Color of Spectral line violet Helium, Neon, and Mercury Discharge Tubes Helium Color of Spectral line Wavelength, λ (nm) 1 2 3 4 Mercury Neon Color of Spectral line C. Identification of the Element in Fluorescent Lights What element is inside fluorescent lights? Hydrogen || Helium || Neon || Mercury (Circle One) Colored Lights and Lasers Incandescent – Green – Black – Red – Blue – Red Laser – Experiment 30 Wavelength, λ (nm) 1 2 3 4 1 2 3 4 D. Color of Spectral line Wavelength, λ (nm) Fluorescent Light Color of Spectral line Wavelength (λ) (nm) 1 2 3 4 30-11 E. F. G. Flame Tests Calcium Nitrate – Barium Nitrate – Sodium Nitrate – Potassium Nitrate – Colorimeter Solution Blue : Yellow Ratio 1 1:1 2 2:1 3 3:1 4 4:1 5 5:1 Absorbance Determining the Concentration of an Unknown Unknown Number Unknown’s Absorbance Experiment 30 30-12