Valence Electrons & Dot Structures Worksheet
advertisement

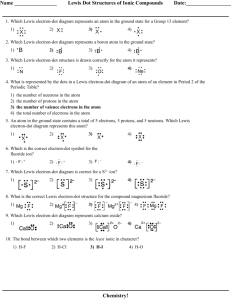
Name _____________ Valence Electrons and Dot Structures Date: ________ 1. What is the total number of valence electrons in a germanium atom in the ground state? 1) 22 2) 2 3) 32 4) 4 2. What is the total number of valence electrons in a calcium atom in the ground state? 1) 8 2) 2 3) 18 4) 20 3. An atom in the ground state contains a total of 5 electrons, 5 protons, and 5 neutrons. Which Lewis electron-dot diagram represents this atom? 1) 2) 3) 4) 4. Which is the electron-dot symbol for an atom with an electron configuration of 2-5? 1) 2) 3) 4) 5. If the electron configuration of an atom of element X is 2-6, the electron dot symbol for the element is 1) 2) 3) 4) 6. Which Lewis electron-dot diagram represents an atom in the ground state for a Group 13 element? 1) 2) 3) 4) 7. The electron-dot symbol X: would best represent 1) Na 2) Mg 3) Cl 4) Ne 8. If ·X: represents the electron-dot symbol of an element, that element could be 1) C 2) O 3) B 4) N 9. An atom in the ground state has seven valence electrons. This atom could be an atom of element? 1) calcium 2) fluorine 3) oxygen 4) sodium 10. Magnesium and calcium have similar chemical properties because a magnesium atom and a calcium atom have the same 1) atomic number 3) total number of electron shells 2) mass number 4) total number of valence electrons Answer Key Valence Electrons and Dot Structures 1. 4 2. 2 3. 3 4. 2 5. 4 6. 3 7. 2 8. 3 9. 2 10. 4