4. Identification of Hydrocarbons Lab
advertisement

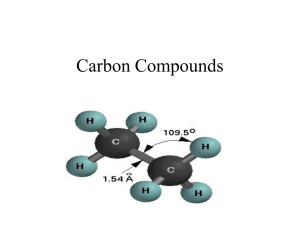
Name: Reactions of Hydrocarbons Properties and Identification of Hydrocarbons Purpose: a) To identify saturated and unsaturated hydrocarbons using properties and reactions. b) Study substitution and addition reactions. Introduction Hydrocarbons, compounds which contain only carbon and hydrogen, can be classified into several types, depending on their structure. Aliphatic hydrocarbons are divided into three classes: - alkanes have only single bonds, and are said to be saturated - alkenes and alkynes have carbon-carbon double or triple bonds, and are said to be unsaturated. - Aromatic hydrocarbons are cyclic compounds whose structure is related to that of benzene, with six -electrons in a six-member ring. Aliphatic Hydrocarbons such as Alkanes are relatively inert to chemical oxidizing agents such as neutral or alkaline permanganate, where alkenes are readily oxidized at room temperature. The change in color can be used as a test for a double bond, provided the molecule contains no easily oxidizable group. Aromatic Hydrocarbons such as toluene will be used in each of the following experiments. Although formally unsaturated, C6H6CH3 in the sense that it has multiple carbon-carbon double bonds, toluene does not give the usual reactions expected of an alkene. It is not easily oxidized, and preferably undergoes substitution rather than addition reactions. Permanganate converts cyclohexene into a diol. Since a syn-hydroxylation takes place, the reaction is thought to involve the formation of an intermediate cyclic manganate ester, which is readily hydrolyzed under the reaction conditions to yield the glycol. In the course of the reaction purple permanganate is reduced to brown manganese dioxide (MnO2). Also this reaction is used as a qualitative test for the presence of an alkene (Baeyer's Test ). The following experiments illustrate some of the fundamental reactions of saturated, unsaturated, and aromatic hydrocarbons. The three classes sometimes react differently toward the same reagent, in which case it may be used to distinguish between them. 1 Name: Materials Each group lab apron eye protection 4 – 13x100mm test tubes Test tube rack 1 – 100mL beaker Communal (teacher monitored) 2 Alkanes or cycloalkanes 2 alkenes 2-4 unknowns hydrocarbons (saturated & unsaturated) conc. H2SO4 1% KMnO4 Experimental Procedure Perform the tests (A) , (B), (C) , and (D) on the following materials 1. The saturated hydrocarbon (alkanes and cycloalkanes) – cycloalkane, pentane, and heptane 2. The unsaturated hydrocarbon (alkenes and alkynes) – cycloalkene and 2-pentene 3. The aromatic hydrocarbon - toluene and benzene A. Aqueous Potassium Permanganate (Baeyer's Test) – In a small test tube, add 5 drops of a hydrocarbon. Carefully add 1% the aqueous KMnO4 solution., dropwise with shaking. Keep track in your notebook the number of drops added and any color changes, or other observations; but do not add more than ten drops of KMnO4 solution. B. Sulfuric Acid Test – In a small test tube add 1 ml of hydrocarbon, cautiously and with gentle shaking, to about 3 ml of concentrated sulfuric acid. Shake the tubes well and note the results. Observe whether heat evolved and whether the hydrocarbon dissolves. Discard the contents by pouring them into a beaker containing at least 50 mL of water. a. Although alkanes are inert to cold, concentrated sulfuric acid, alkenes react by addition. The product, alkyl hydrogen sulfate, is soluble in concentrated sulfuric acid. C. Solubility – Add about 2 ml. of water in a small test tube and add 2 or 3 drops of the hydrocarbon to be tested. Shake the mixture to determine whether the hydrocarbon is soluble (a colorless second layer may be hard to see). Record your results a. This confirms (for the most part) that the compound is indeed organic. DISPOSAL: Please dispose all waste from these test tubes experiments in the appropriate waste container as directed by instructor. 2 Name: Identification of Unknowns: By comparing the observations you made for your unknowns with those of the known hydrocarbons, you can identify classify unknowns A and B as alkanes, alkenes or aromatic compounds. Record their classifications and offer an explanation for your choice. Results: OBSERVATIONS ON HYDROCARBONS Hydrocarbon Tests Hydrocarbon 1% KMnO4 Conc. H2SO4 Alkane Alkane Alkene Alkene Unknown #___ Unknown #___ Results: Unknown # Classification (saturated, unsaturated) 3 H2O (solubility) Name: Lab Questions: 1. Draw condensed structure formulas for the following compounds. cyclohexane cyclohexene toluene 2. What colors are potassium permanganate solutions? 3. What are the general formulas for alkenes and cycloalkenes? 4. What did you determine your unknown solutions to be? 5. How could you distinguish octanol from 1-octene by a simple chemical test? 6. What would you expect the difference between reactivity of a) hexane and Cyclohexane b) hexane and cyclohexene? Explain your answers. 4