here
advertisement

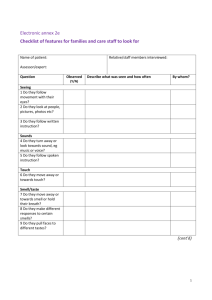
Report: Organic Reactions Hopefully here for the Report Form Note: In preparing this report you are free to use references and consult with others. However, you may not copy from other students’ work (including your laboratory partner) or misrepresent your own data (see honor code). Name(Print then sign): ___________________________________________________ Lab Day: ___________________Section: ________TA__________________________ Observations: Synthesis of esters Reagents C 7 H 6 O 3 (salicylic acid ) + CH3OH (methyl alcohol) CH 3CH 2 CH 2 CH 2 CH 2 CH 2 CH 2 CH 2 OH (1 - octanol ) CH 3 COOH (glacial acetic acid) CH 3 CH 2 CH 2 CH 2 CH 2 OH (amyl alcohol) CH 3 COOH (glacial acetic acid) C 2 H 5 OH (ethanol) CH 3 COOH (acetic acid) Product Observations Oxidation of an alcohol with acidified potassium dichromate(VI) solution. Remember to describe what happens and explain the color changes. What conditions and techniques would favour the oxidation of ethanol to a. ethanal rather than ethanoic acid. b. ethanoic acid rather than ethanal? Observations: Observations: Add 10 drops of dil. H 2SO 4 to 5 drops of color/smell color/smell on warming K 2 Cr2 O 7 to the following alcohols Smell cautiously! Smell cautiously! Ethanol Methanol Propan-2-ol Oxidation of an alcohol with acidified potassium permanganate (VII) solution Remember to describe what happens and explain the color changes. What is your final product? Observations: Observations: color/smell Add 10 drops of dil. H 2SO 4 to 5 drops of color/smell on warming KMnO 4 to the following alcohols Smell cautiously! Smell cautiously! Ethanol Methanol Propan-2-ol Saponification of a vegetable oil Reagent Your soap Laundry Detergent Hand Soap pH from indicator paper