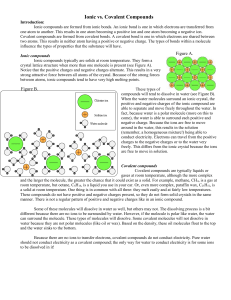
Name: _____________________ Exam 2: Chemical vs. Physical Changes Draw the following ionic bonds using a Lewis Dot Structure. (9 points) K+F Mg + I Be + S Draw the following covalent bonds. (9 points) F+F C+O H+O Name the following ionic compounds. (6 points) 1. NaCl: _____________________________________________________________________ 2. FeS: ______________________________________________________________________ 3. CaCO3 : ____________________________________________________________________ Name the following binary covalent compounds. (6 points) 1. SO2 : ______________________________________________________________________ 2. N2 O: _______________________________________________________________________ 3. H2 O: _______________________________________________________________________ In the boxes below, please draw a particle model for water in a solid, liquid, and gas state. (3 points) Solid Liquid Gas Below is a list of ionic vs. molecular compound properties. Assign the properties to the correct side of the chart. (12 Points) Ionic Compounds • Transfers Electrons Between Atoms • Between Atoms Molecular Compounds • Low Boiling Point • Good Conductivity • Nonmetals Only • Shares pairs of Electrons • High Boiling Point • Typically High Solubility • Only Solids at Room Temperature • Poor Conductivity • Gas, Liquid, or Solid at Room Temperature • Variable Solubility • Metal vs. Nonmetal What mass of solute will dissolve in 100mL of water at the following temperatures? (4 points) a. KNO at 70° = ____________ b. NaCl at 100°C = ____________ c. NH at 90°C = ____________ d. Which of the above substances is most soluble in water at °C 15? = ____________