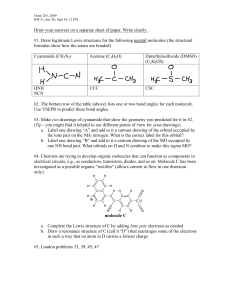
Question 1: Draw the Lewis Dot structure of CO2 and H2O. Analyze
advertisement
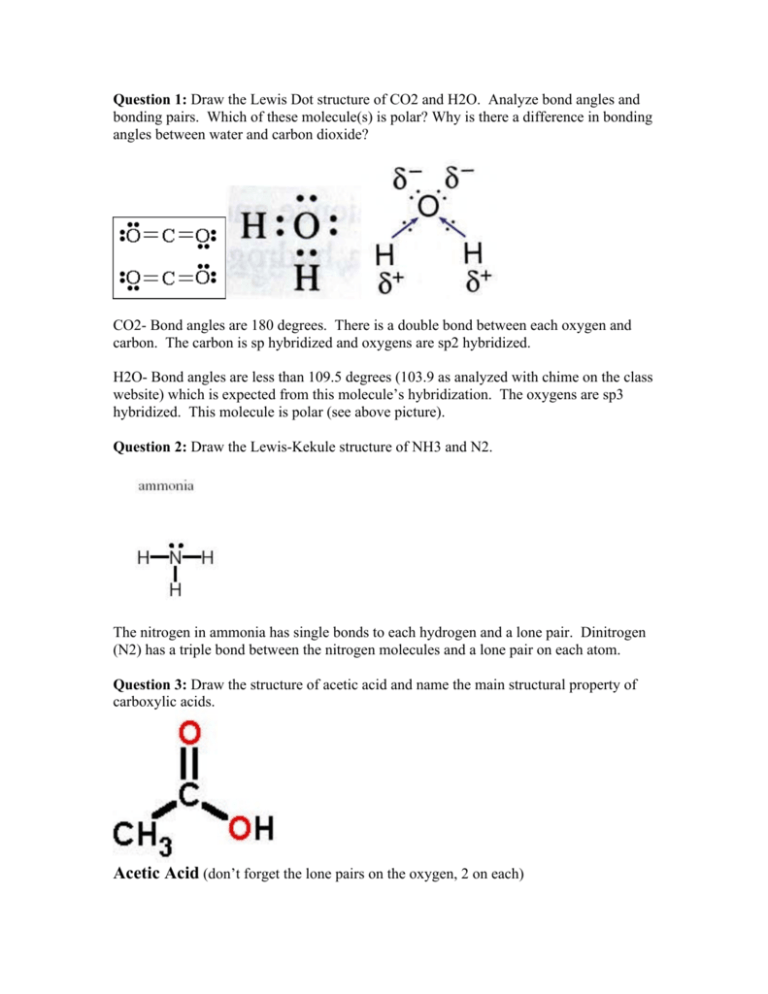
Question 1: Draw the Lewis Dot structure of CO2 and H2O. Analyze bond angles and bonding pairs. Which of these molecule(s) is polar? Why is there a difference in bonding angles between water and carbon dioxide? CO2- Bond angles are 180 degrees. There is a double bond between each oxygen and carbon. The carbon is sp hybridized and oxygens are sp2 hybridized. H2O- Bond angles are less than 109.5 degrees (103.9 as analyzed with chime on the class website) which is expected from this molecule’s hybridization. The oxygens are sp3 hybridized. This molecule is polar (see above picture). Question 2: Draw the Lewis-Kekule structure of NH3 and N2. The nitrogen in ammonia has single bonds to each hydrogen and a lone pair. Dinitrogen (N2) has a triple bond between the nitrogen molecules and a lone pair on each atom. Question 3: Draw the structure of acetic acid and name the main structural property of carboxylic acids. Acetic Acid (don’t forget the lone pairs on the oxygen, 2 on each) Properties of carboxylic acids include R-COOH general structure. Notice that there is always one double bonded oxygen and one hydroxyl group. Question 4: The gas from the hydrothermal vents is hot before the seawater cools it. What does this say about the energy of and the molecules released by the vents? Based on this and the article, would you say the first reactions to proceed to life were endothermic or exothermic? The molecules released by the vents are high in energy. This means that the first reactions that proceeded life must have been endothermic because they took the energy away from the water that surrounded them. Question 5: What is the iron sulfur theory and what is the purpose of catalysts in reactions? (from article) "the iron sulfur world theory," because he believes that metallic surfaces, particularly those in iron sulfide, "would have been promising facilitators or catalysts that created the precursor chemicals of living cells" The purpose of catalysts is to lower the activation energy of the reaction. This means that there needs to be less energy present for the reaction to move forward and create the products. Question 6: Draw the structure of pyruvic acid. Give the chemical formula and the common name of the molecule added to create alanine from pyruvic acid. (HINT: See Insert Above) Pyruvic Acid NH3 common name “ammonia” is added to pyruvic acid to create alanine.