Fe + SCN – W FeSCN
advertisement

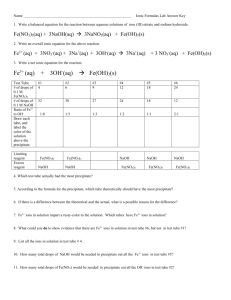
Lab 9.3 Analysis Questions (Key) Fe3+ + SCN – 1. W FeSCN2+ colorless red What substance did you add to the beaker that provided the Fe3+ ions? The Fe(NO3)3 solution provided Fe3+ ions. 2. Write the dissociation equation showing the formation of Fe3+ ions. Fe(NO3)3 ö Fe3+ (aq) + 3 NO3– (aq) 3. What substance did you add to the beaker that provided the SCN – ions? The KSCN solution provided SCN– ions. 4. Write the dissociation equation showing the formation of SCN – ions. KSCN ö K+ (aq) + SCN– (aq) 5. To the second test tube you added 3 drops of 0.1 M KSCN. a. What was the “important” ion that you were adding to the equilibrium reaction? SCN– is the ion which matters since it appears in the equilibrium equation at the top of the page. b. How did the color of the second test tube compare to that of the control? Explain why this occurred. The second tube was a darker reddish color than the control. This could only happen if the redcolored FeSCN 2+ ion became more concentrated. c. Using the equilibrium equation as a guide, identify how the concentration of each ion changed. Initially the SCN– concentration increased due to the disturbance of adding KSCN solution (shown below in green). The reaction compensated by shifting right, combining some of the added SCN – with Fe3+ ions already in solution to produce more FeSCN 2+ ions. The shift thus decreased SCN– and Fe3+ concentrations and increased the FeSCN2+ concentration (shown below in red). Fe3+ + 9 6. SCN– W 89 FeSCN2+ 8 To the third test tube you added 3 drops of 0.2 M Fe(NO 3) 3. a. What was the "important" ion that you were adding to the equilibrium reaction? Fe3+ is the ion which matters since it appears in the equilibrium equation. b. How did the color of the third test tube compare to that of the control? Explain why this occurred. The third tube was a darker reddish color than the control. Again, the red-colored FeSCN 2+ ion must have became more concentrated. c. Using the equilibrium equation as a guide, identify how the concentration of each ion changed. Initially the Fe3+ concentration increased due to the disturbance of adding Fe(NO3)3 solution (shown in green). The reaction compensated by shifting right, combining some of the added Fe3+ with SCN– ions already in solution to produce more FeSCN 2+ ions. The shift thus decreased SCN– and Fe3+ concentrations and increased the FeSCN2+ concentration (shown in red). Fe3+ + 89 7. SCN– W 9 FeSCN2+ 8 To the fourth test tube, you added 5 drops of 0.1 M Na2HPO 4. This substance dissociated to produce HPO 42– ions. This ion, HPO 42–, will react with Fe3+ ions to form a precipitate. a. How did the color in the fourth test tube compare to that of the control? What happened to the FeSCN 2+ concentration? The fourth tube was virtually colorless—certainly a much lighter in color than the control. The red-colored FeSCN 2+ ion must have became much less concentrated. b. Given the information about the behavior of the HPO 42– ion and you r know ledge o f Le Châtelie r’s Principle, explain why the color of the reaction changed the way it did. Initially the Fe3+ concentration decreased due to the disturbance of HPO42– ions trapping iron ions as solid precipitate (shown in green). The reaction compensated by shifting left, splitting FeSCN 2+ ions into Fe3+ and SCN– ions in an attempt to replace missing Fe3+ ions. The shift thus increased Fe3+ and SCN– concentrations and decreased the FeSCN2+ concentration (shown in red). Fe3+ + 98 SCN– W 8 FeSCN2+ 9