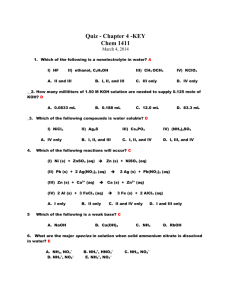
Name Ionic Formulas Lab Answer Key 1. Write a balanced equation
advertisement
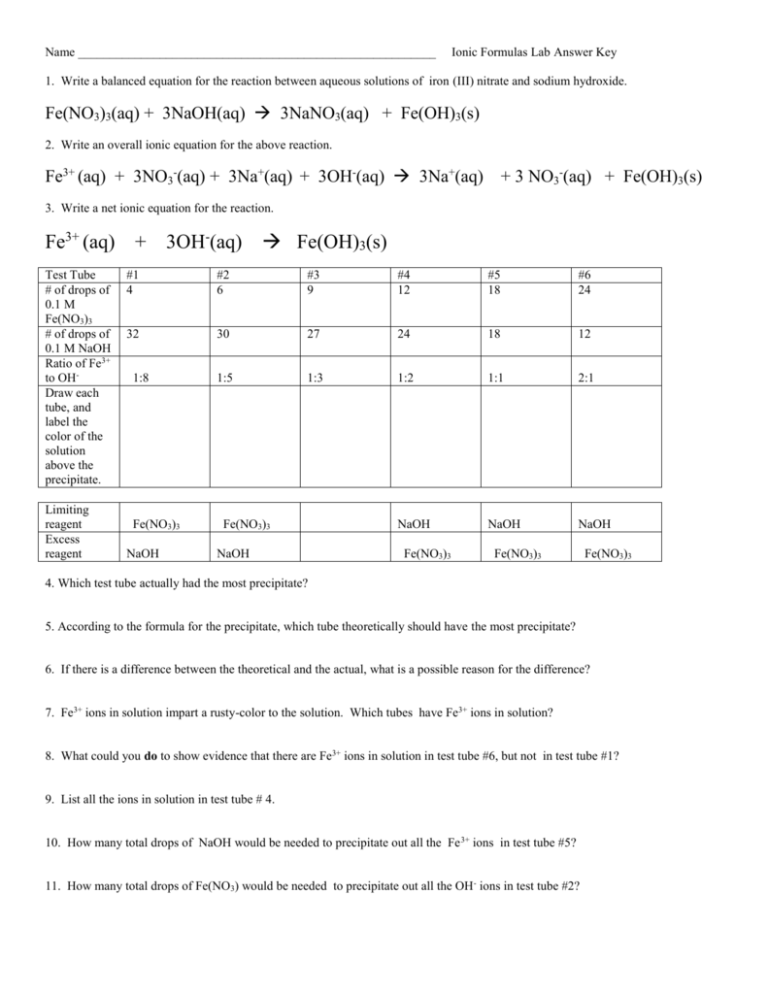
Name _________________________________________________________ Ionic Formulas Lab Answer Key 1. Write a balanced equation for the reaction between aqueous solutions of iron (III) nitrate and sodium hydroxide. Fe(NO3)3(aq) + 3NaOH(aq) 3NaNO3(aq) + Fe(OH)3(s) 2. Write an overall ionic equation for the above reaction. Fe3+ (aq) + 3NO3-(aq) + 3Na+(aq) + 3OH-(aq) 3Na+(aq) + 3 NO3-(aq) + Fe(OH)3(s) 3. Write a net ionic equation for the reaction. Fe3+ (aq) + 3OH-(aq) Test Tube # of drops of 0.1 M Fe(NO3)3 # of drops of 0.1 M NaOH Ratio of Fe3+ to OHDraw each tube, and label the color of the solution above the precipitate. Limiting reagent Excess reagent Fe(OH)3(s) #1 4 #2 6 #3 9 #4 12 #5 18 #6 24 32 30 27 24 18 12 1:5 1:3 1:2 1:1 2:1 NaOH NaOH NaOH 1:8 Fe(NO3)3 NaOH Fe(NO3)3 NaOH Fe(NO3)3 Fe(NO3)3 Fe(NO3)3 4. Which test tube actually had the most precipitate? 5. According to the formula for the precipitate, which tube theoretically should have the most precipitate? 6. If there is a difference between the theoretical and the actual, what is a possible reason for the difference? 7. Fe3+ ions in solution impart a rusty-color to the solution. Which tubes have Fe3+ ions in solution? 8. What could you do to show evidence that there are Fe3+ ions in solution in test tube #6, but not in test tube #1? 9. List all the ions in solution in test tube # 4. 10. How many total drops of NaOH would be needed to precipitate out all the Fe 3+ ions in test tube #5? 11. How many total drops of Fe(NO3) would be needed to precipitate out all the OH- ions in test tube #2?