Exam 2 Material Outline MS Word
advertisement

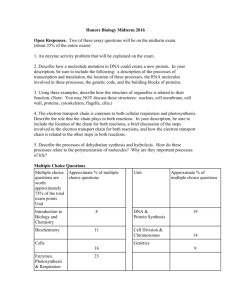
Bio 1 General Biology – Exam 2 Outline Powering Life: Energy (Chapter 6) How is a cell able to perform all of its functions to survive? I. Energy A. Two forms of energy 1. Potential energy Examples? 2. Kinetic energy Examples? Energy can be changed from one form to another! Ex. Food = potential energy which changes into kinetic energy when organism moves around or grows B. Thermodynamics – the study of energy and its transformations 1. First Law of Thermodynamics (Conservation of Energy) a. Organisms cannot create energy that it needs to live. It must capture it from the environment and change it into a usable form. Ex. Photosynthesis –uses sunlight energy and converts it to chemical energy contained in the bonds in sugars that plant produces. Ex. Animals eat food, which is transformed to chemical energy, which is transformed into mechanical energy, muscle movement etc. 2. Second Law of Thermodynamics a. Whenever energy is converted, some useable energy is degraded into a less useable and ordered form (heat). We are not losing energy just changing it to a less useable form. Ex. Food is used for movement which releases heat energy (less usable form) b. No process involving energy conversion is 100% efficient. Why? As a result, what happens to the amount of useable energy over time? 3. The Laws of Thermodynamics and Us a. What do Arctic peoples tend to produce more of? b. What is the upside of this extra production? c. What is the downside of this extra production? View course website animations on energy D. Measuring Energy 1. Calorie Ex. Can measure caloric content of a peanut (or any food) by burning it under a container of water to convert all of the stored chemical energy to heat and then measure the temperature increase of the water 2. Kilocalories (kcal) – units of 1,000 calories; calories on food packages are actually kilocalories Ex. One peanut has 5 food calories (5 kcal) which is enough energy to increase temp. of 1 kg (a little more than a quart) of water by 5º C. A handful would boil 1 kg of water. II. How Energy Is Used By Living Things (The Energy Molecule: ATP) Carbs, fats and other fuel molecules we get from food do not drive work in our cells. They must be broken down so that their energy is released. Chemical energy (ATP) is released by the breakdown of organic molecules during cellular respiration. In what organelle does ATP production occur? ATP then powers cellular work. A. ATP (Adenosine triphosphate) – the energy molecule (Fig. 6.6) 1. 3 phosphate groups each with a negative charge. 2. When energy is needed, ATP is broken down, a phosphate is removed, & energy is released. 3. When cell needs a place to store energy, a phosphate is added back on. B. Renewable ATP (Fig. 6.6) 1. A working cell recycles all of its ATP about once each minute. That is about 10 million ATP molecules spent and regenerated per second per cell. 2. ATP can be restored by adding a phosphate group back to ADP. 3. This takes energy; like recompressing a spring 4. This is where food enters the story. Chemical energy that cellular respiration harvests from food is put to work regenerating the cell’s supply of ATP. III. Using Energy Efficiently with Enzymes Read pp. 115-116: “Efficient Energy Use in Living Things: Enzymes” and answer the following: A. What is an enzyme? B. What is our metabolism and how are enzymes involved in our metabolism? C. How Enzymes Work (Fig. 6.9) 1. Enzymes enable reactions to occur faster by reducing the amount of energy required to get reactions going. 2. Enzymes bind to their substrate which makes the substrate more vulnerable to chemical alteration. 3. Each enzyme is specific to the reaction it speeds up (> 5000 enzymes exist) Ex. Lactase = enzyme that breaks down lactose (milk sugar) 4. Vitamins help enzymes bind to their substrates. View course website animations on enzymes. D. What will stop enzymes from working? Energy for Cells: Cellular Respiration (Chapter 7) How Cells Gain Energy (ATP) From Food: This is done by a process called cellular respiration I. Aerobic Cellular Respiration – how cells harvest energy (ATP) from organic molecules (food) using oxygen A. General Characteristics: 1. Cellular respiration requires cells to take in oxygen and glucose and release wastes in the form of carbon dioxide and water. Cellular respiration is the reason why we breathe and eat. B. Why is oxygen important to aerobic cellular respiration? 1. Equation for cellular respiration: Where did the hydrogens (electrons) in glucose go? 2. These electrons (hydrogens) hold energy (high energy electrons) What type of energy are they holding, potential or kinetic? 3. When high energy electrons (hydrogens) change partners, from sugar to oxygen, the energy is released a. Electrons (hydrogens) are strongly attracted to oxygen. 4. How do high energy electrons (hydrogens) make their way from glucose to oxygen? (Fig. 7.3) a. Electrons (hydrogens) are carried to oxygen by electron carriers called NAD+ and FAD b. NAD+ (nicotinamide adenine dinucleotide) accepts electron (hydrogen) from glucose and becomes NADH. FAD accepts electrons (hydrogens) and becomes FADH2 c. Electrons carried by electron carriers are taken to the electron transport chain (the last step in aerobic cellular respiration) d. Oxygen pulls the energy filled electrons through the electron transport chain and the energy is released. C. The Mitochondria – where cellular respiration (energy production) occurs 1. Mitochondria Anatomy: a. Cristae b. Matrix D. 4 Steps of Cellular Respiration: 1) Glycolysis, 2) Intermediate Step, 3) Krebs (Citric Acid) Cycle and 4) the Electron Transport Chain (ETC) (Fig. 7.4) All four steps together produce 36 - 38 ATP! - Glycolysis = more ancient; least efficient step (found in all organisms) - Other 3 steps = evolved later; are more efficient (not found in all organisms) 1. Glycolysis ‘sugar splitting’ (Fig. 7.5) a. Glucose enters cell from bloodstream and is broken down into 2 pyruvates b. Electrons (hydrogens) are removed by electron carriers NAD+ forming NADH (high energy electron) c. 4 ATP are produced and 2 ATP are used producing a net gain of 2 ATP d. Location of this step? INPUTS: OUTPUTS: View course website animations on Glycolysis 2. Intermediate Step (Fig. 7.7) a. Each pyruvate is broken down further into Acetyl CoA b. Electron (hydrogen) is removed by electron carrier NAD+ forming NADH (high energy electron) c. CO2 is released d. Location of this step? INPUTS: OUTPUTS: 3. Krebs (Citric Acid) Cycle (Fig. 7.8) a. Acetyl CoA is broken down and CO2 is produced and released b. Electrons (hydrogens) are removed by electron carriers NAD+ & FAD forming NADH and FADH2 (high energy electrons) c. 2 ATP are produced d. Location of this step? INPUTS: OUTPUTS: View course website animations on the Krebs (Citric Acid) Cycle 4. Electron Transport Chain (Fig. 7.9) (bulk of ATP is produced here) a. Receives the high energy electrons (hydrogens) from NADH and FADH2 (produced from previous steps) b. Electrons (hydrogens) are run along enzymes c. This powers the process of the 3rd phosphate group to attach to ADP to make ATP (recompressing the spring) producing 32-34 ATP d. Electrons (hydrogens) are finally accepted by oxygen (the final electron acceptor) producing water e. Location of this step? INPUTS: OUTPUTS: View course website animation on the Electron Transport Chain It takes about 10 million ATP molecules per second to power an active muscle cell! Aerobic Cellular Respiration Review E. Energy From Food: We concentrated on the breakdown of glucose but cellular respiration also breaks down other foods to produce ATP (Fig. 7.10) View course website animation on Cellular Respiration II. Anaerobic Cellular Respiration (Fermentation) - the harvesting of chemical energy (ATP) from organic fuel molecules (food) without using oxygen What step in aerobic cellular respiration does not require oxygen? Does this step produce a lot or little ATP? What kind of organisms can do this and why would they do this? A. Fermentation in Microbes 1. Yeast – (a single-celled fungus) can live by glycolysis alone if it is placed in an environment that lacks oxygen a. What waste products are produced when yeasts only use glycolysis to produce their ATP? b. How do we take advantage of these waste products? B. Fermentation in Animal Muscle Cells 1. During quick bursts of energy use, oxygen cannot be delivered into muscle cells fast enough, so they turn to glycolysis to supply ATP. 2. What waste product is produced when muscle cells only use glycolysis to produce their ATP? lactate Cellular Respiration & Metabolic Rates (Activity Level) Different types of animals will undergo cellular respiration and produce energy at different rates. And, their activity levels will depend on how fast they produce energy. I. Ectothermic animals (ecto = outside; therm = heat) - internal body temperature is controlled by temperature of environment A. Examples?? B. Ectothermic animal characteristics: 1. Are they able to internally regulate their body temperature? 2. Do they exist at same temperature as their surroundings? 3. How do they obtain their body heat? 4. How is metabolic rate (activity level) affected when environmental temperature changes? 5. How will the process of cellular respiration be affected when it is in a warm environment? When in a cold environment? II. Endothermic animals (endo = inside; therm = heat) - internal temperature is controlled by internal metabolism A. Examples? B. Endothermic animal characteristics: 1. Are they able to internally regulate their body temperature? 2. Do they exist at same temperature as their surroundings? 3. How do they obtain their body heat? 4. How is metabolic rate (activity level) affected when environmental temperature changes? 5. How will the process of cellular respiration be affected when it is in a warm environment? When in a cold environment? III. Advantages & Disadvantages of Being Ectothermic IV. Advantages & Disadvantages of Being Endothermic Energy for Life: Photosynthesis (Chapter 8) I. Autotrophs vs. Heterotrophs A. Autotrophs (producers) – “self-feeders” B. Heterotrophs (consumers & decomposers) – “other-feeders” II. Photosynthesis – the process that transforms simple molecules (CO2) into more complex molecules (carbohydrates) using H2O, minerals and sunlight Carbon dioxide and water are the end products of what process? Where did the hydrogens (electrons) in water go? How is energy being transformed during photosynthesis? What is the fate of glucose produced from photosynthesis? A. Components of Photosynthesis 1. Photosynthesis is driven by visible light (Fig. 8.3) a. Why are plants green? - What colors of light are absorbed? - What color of light is reflected? 2. Chloroplasts – organelles where photosynthesis occurs (Fig. 8.4) a. Thylakoids b. Stroma 3. The Leaf (Fig. 8.4) a. Stomata Where are stomata located? B. 2 Stages of Photosynthesis: (Fig. 8.9) 1. Light Reactions (“photo” part of photosynththesis) (Fig. 8.7): converts solar energy to chemical energy H2O + light energy O2 + electron (hydrogen) carriers (NADPH) + ATP Location of this step? INPUTS: OUPUTS: View course website animation on the Light Reactions 2. Calvin Cycle Reactions (“synthesis” part of photosynthesis) (Fig. 8.8): makes glucose from CO2 electron carriers (NADPH) + ATP + CO2 glucose Location of this step? INPUTS: OUPUTS: View course website animation on the Calvin Cycle and on Photosynthesis Photosynthesis produces our planet’s oxygen as well as 155 billion tons of biomass each year (about 25 tons for every person on earth)! C. Variations of Photosynthesis in Different Climates Read p149-152 “Photorespiration and the C4 Pathway” and p. 152-153 “CAM Photosynthesis” and visit the website http://wc.pima.edu/~bfiero/tucsonecology/plants/plants_photosynthesis.htm to answer the following questions: 1. What necessary molecule does the enzyme rubisco capture from the atmosphere for plants? 2. What unnecessary molecule will rubisco sometimes capture instead and what is this process known as? 3. Briefly state 1) when C3 plants open their stomata, 2) the adaptive value of C3 photosynthesis 3) the problems with C3 photosynthesis when dealing with water loss and photorespiration in warm climates and 4) the types of plants that use this process. 4. Briefly state 1) when C4 plants open their stomata, 2) the adaptive value of C4 photosynthesis when dealing with water loss and photorespiration in warm climates and 3) the types of plants that use this process. 5. Briefly state 1) when CAM plants open their stomata, 2) the adaptive value of CAM photosynthesis when dealing with water loss in hot, dry climates and 3) the types of plants that use this process. D. Using Photosynthesis to Fight Climate Change 1. Read p. 150-151 “Using Photosynthesis to Fight Global Warming” and answer the following: a. Why are trees known as carbon sinks? b. Describe a possible way to increase photosynthesis worldwide. c. Describe a possible way to lock up the products of photosynthesis so that they don’t decay and then don’t release carbon dioxide. d. Describe two other possible carbon capture ideas. 2. Watch the video called “Capturing Carbon” and answer the following: http://www.pbs.org/wgbh/nova/sciencenow/0302/03.html a. Briefly describe this “green machine” that has been invented and state what it does. b. What problem can this invention help solve? 3. Watch the video called “Algae Fuel” and answer the following: http://www.pbs.org/wgbh/nova/sciencenow/0406/02.html a. Photosynthesizing algae produce oxygen, sugar, and what other product which can be converted into biodiesel (biofuel)? b. What are the two major disadvantages of producing corn based ethanol as biofuel? c. What is the advantage of producing algae for biofuel? d. What is the disadvantage of producing algae for biofuel? III. The Big Picture: Energy flow in living things DNA & Protein Synthesis (Chapter 13 & 14) I. The Discovery of the Double Helix A. Prior to the discovery of DNA structure: 1. By 1920 – knew that genetic info resided on chromosomes but nobody could really say what a gene looked like and how it exactly worked 2. By 1930’s & 40’s knew that genes bring about the production of proteins 3. By 1940’s & 50’s knew that genes are composed of deoxyribonucleic acid (DNA) but did not know how it carried out its function or how DNA can be copied. B. The Scientists Involved in the Race to Discover the Structure of DNA: 1. Linus Pauling of Caltech: was most likely to solve DNA structure. He studied chemistry of large organic molecules and was an expert in X-ray crystallography (diffraction) (bombarding crystals of organic molecules such as DNA with X-rays and recorded how X-rays bounced off molecules. 2. Rosalind Franklin and Maurice Wilkins: researchers from King’s College London also experts in X-ray diffraction and had good idea of general shape DNA but their approach was slow. 3. James Watson (23 year old American) & Francis Crick (35 year old Englishman): met in Cambridge University, England in 1951. Did not have expertise on chemical bonds or X-ray crystallography like the others and did no experiments like the other two. Built models based off of Franklin’s and Wilkin’s data and pictures. C. The Discovery and After: 1. In 1953, Watson and Crick discovered the double helix structure. 2. In 1958 at the age of 37 Rosalind Franklin died 3. In 1962 Watson, Crick & Wilkins were awarded he Nobel Prize for Medicine or Physiology. II. Deoxyribonucleic Acid (DNA) Structure (Fig. 13.5) A. DNA Parts: 1) Phosphate group 2) Deoxyribose (sugar) 3) Bases: adenine (A), guanine (G), thymine (T) or cytosine (C) What bases pair up? B. DNA Replication (How DNA makes a copy of itself) (Fig. 13.6) Do you see a template? Because of the variety of base pairs, DNA is versatile enough to specify the array of proteins that exists when strung together by the thousands. View course website animation on DNA Replication III. How is this genetic information used? A. Genotype B. Phenotype Does genotype largely determine phenotype? C. Relationship between genotype and protein molecules that determine the phenotype: 1. Gene IV. How Proteins Are Made A. Protein structure (fig. 14.1) 1. Monomer unit of a protein? 2. What is a protein polymer called? 3. When is a protein considered a “working protein”? 2 steps of protein synthesis: B. Transcription – process by which genetic info in DNA is copied onto messenger RNA (mRNA) (Fig. 14.2 & 14.4) 1. Where does transcription occur in a cell? 2. DNA is unwound and complementary RNA nucleotides pair up with DNA strand to form a copy called messenger RNA (mRNA). What does Adenine on DNA strand link with on RNA strand? 3. Three mRNA bases (codon) specifies a single amino acid (Fig. 14.5) View course website animation on Transcription C. Translation – process by which info encoded in mRNA is used to assemble a protein at a ribosome (Fig. 14.2, 14.6, 14.7, 14.8, 14.9) 1. Where does translation occur in a cell? 2. Transfer RNA (tRNA) brings amino acids to ribosome to be strung together in the order specified by mRNA codons View course website animation on Translation Protein Synthesis: Transcription & Translation View course website animation on Protein Synthesis: All Steps TIME TO PRACTICE! The following is a DNA strand (gene) that contains the instructions for building a specific protein: TACGTTGCGATACCTGGGATT 1. Transcribe this gene by writing out its correct mRNA strand. 2. Where does this step occur in a cell? 3. Now translate the mRNA strand that you have just made by using the genetic code below. You are building an amino acid chain (polypeptide)! You can just write the abbreviations for each amino acid. For this exercise we will not use tRNA but keep in mind tRNA would bring the correct amino acid in by pairing its anti-codon to each codon. 4. Where does this step occur in a cell and what organelle is responsible for this step? 3. Once the amino acid chain enters the rough ER and takes its proper shape, it is a working protein! V. The Genetic Code A. What is it? 1. The sequences of bases in mRNA codes for a particular sequence of amino acids in a polypeptide. 2. Triplet codes of mRNA bases specify each of the 20 amino acids B. The genetic code is shared by all organisms, which means the code is universal! Why is this universality of the genetic code important to science? Read p. 254 Essay “The Importance of the Genetic Code” and answer the following: 1. The fact that the code is universal turned out to be important in two ways. The first way increased our knowledge of evolution. In terms of evolution, what is this universality evidence of? 2. The second importance of this universality turned out to have a practical consequence. What does this mean in terms of genes functioning in different organisms? State both a bad and good consequences of this universality. VI. Genome – complete haploid set of an organism’s chromosomes (DNA) - Human Genome Project was completed in 2004. - Human genome is 3.2 billion base pairs long. - Human genome is thought to have 20,000 – 25,000 genes - DNA in just one of our cells would stretch to about 6 ft. in length if uncoiled. Only about 1 in. of this length is DNA that codes for proteins. DNA in all of our cells (~ 100 trillion) would stretch from here to the moon! VII. Gene Mutation A. Types of Mutations 1. Point Mutation (Base Substitutions) Ex. Sickle cell anemia Will point mutations always change the amino acid sequence of protein that is produced? Why or why not? 2. Frameshift Mutation (Nucleotide Insertion/Deletion) What type of mutation has a greater overall effect on protein production and why? B. What causes DNA to mutate? 1. Some mutagens cause cancer. Cancerous growths are cells that have undergone a special kind of mutation that causes the affected cells to reproduce wildly. Once cells start multiplying, they move into normal tissues & destroy ability to function. C. Mutations can be good. How so?