Assignment 3
advertisement

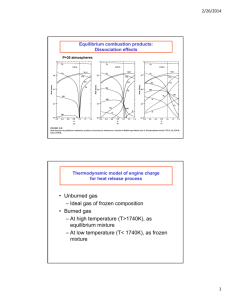
ME444 Fall 2015 Assignment 3 Date Due: 9/24/15 1. Determine the molal analysis of the products of combustion when octane, C8H18 is burned with 300% theoretical air, and determine the dew point of the products if the pressure is 14.7 lbf/in2. 2. Calculate the theoretical air-fuel ratio for the combustion of Octane, C8H18. Do the same calculation for ethanol, C2H5OH. 3. A small air-cooled gasoline engine is tested, and the output is found to be 1.34 hp. The temperature of the products is measured and found to be 730F. The products are analyzed and on a dry volumetric basis were found to be: CO2=14%, CO=2.9%, O2=1.6%, N2=84.1% The fuel is liquid octane and the fuel-air entering the engine is 77F. The fuel flow rate to the engine is 1.20 lbm/hr. Determine the rate of heat transfer from the engine and the efficiency of the engine. 4. Liquid octane at 77F is burned with 300% theoretical air at 77F in a steady flow reactor. Determine the adiabatic flame temperature. 5. Carefully draw a P-V diagram for a 4-stroke cycle IC engine operating at maximum torque. Label all important valve events. Assume this is a modern automotive engine an make the necessary assumptions and use references to find an actual “nominal” value for peak pressure.