Writing Chemical Formulas and Naming Compounds
advertisement
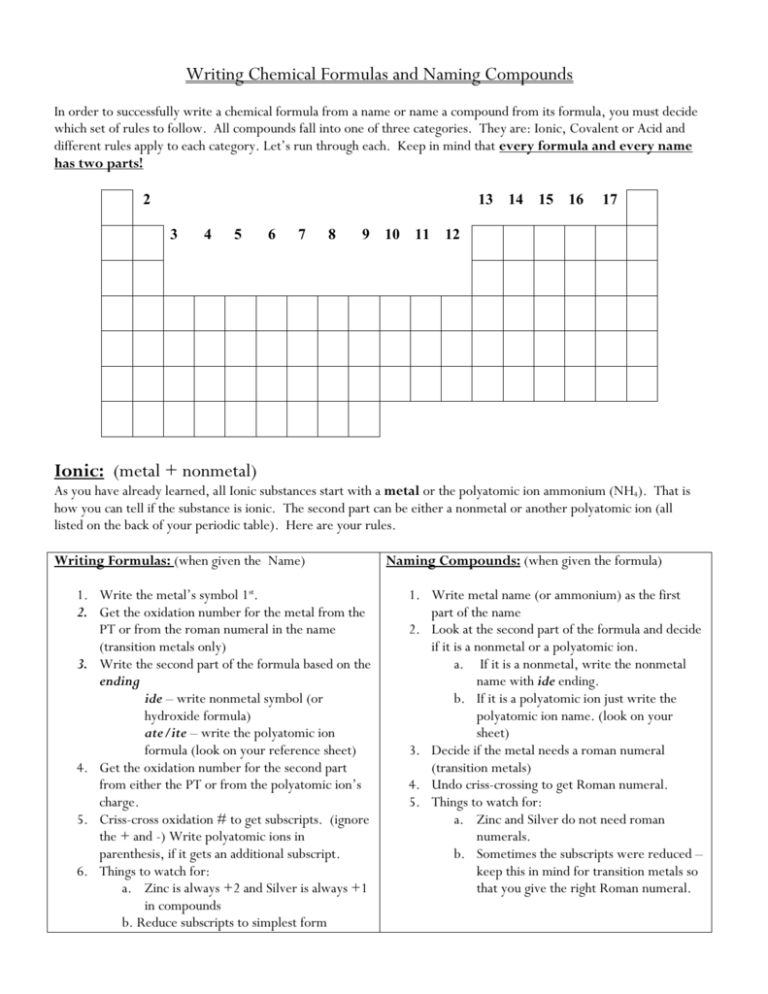
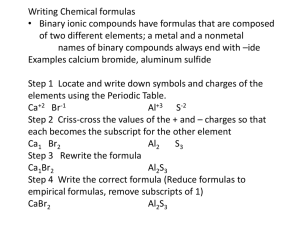
Writing Chemical Formulas and Naming Compounds In order to successfully write a chemical formula from a name or name a compound from its formula, you must decide which set of rules to follow. All compounds fall into one of three categories. They are: Ionic, Covalent or Acid and different rules apply to each category. Let’s run through each. Keep in mind that every formula and every name has two parts! 2 13 3 4 5 6 7 8 9 10 11 14 15 16 17 12 Ionic: (metal + nonmetal) As you have already learned, all Ionic substances start with a metal or the polyatomic ion ammonium (NH4). That is how you can tell if the substance is ionic. The second part can be either a nonmetal or another polyatomic ion (all listed on the back of your periodic table). Here are your rules. Writing Formulas: (when given the Name) 1. Write the metal’s symbol 1st. 2. Get the oxidation number for the metal from the PT or from the roman numeral in the name (transition metals only) 3. Write the second part of the formula based on the ending ide – write nonmetal symbol (or hydroxide formula) ate/ite – write the polyatomic ion formula (look on your reference sheet) 4. Get the oxidation number for the second part from either the PT or from the polyatomic ion’s charge. 5. Criss-cross oxidation # to get subscripts. (ignore the + and -) Write polyatomic ions in parenthesis, if it gets an additional subscript. 6. Things to watch for: a. Zinc is always +2 and Silver is always +1 in compounds b. Reduce subscripts to simplest form Naming Compounds: (when given the formula) 1. Write metal name (or ammonium) as the first part of the name 2. Look at the second part of the formula and decide if it is a nonmetal or a polyatomic ion. a. If it is a nonmetal, write the nonmetal name with ide ending. b. If it is a polyatomic ion just write the polyatomic ion name. (look on your sheet) 3. Decide if the metal needs a roman numeral (transition metals) 4. Undo criss-crossing to get Roman numeral. 5. Things to watch for: a. Zinc and Silver do not need roman numerals. b. Sometimes the subscripts were reduced – keep this in mind for transition metals so that you give the right Roman numeral. Ex. Ex. K2CrO4 Calcium chloride AlCl3 Lithium sulfate MgSO4 Magnesium nitrate Cu(NO3)2 Iron (III) carbonate Covalent (Molecular): (nonmetal + nonmetal) As you have already learned, covalent substances contain only nonmetals. Below are the rules for writing the formula from the chemical name or naming the chemical from the formula. Writing Formula: 1. DO NOT look at oxidation #’s!!!!! 2. For the first part of the formula write the first nonmetal’s symbol and use the prefix to determine its subscript 3. For the second part, write the second nonmetal’s symbol, again using the prefix to get subscript 4. Prefix Meanings: mono or no prefix = 1 di = 2 tri= 3 tetra = 4 penta = 5 hexa = 6 hepta = 7 octa = 8 nona = 9 deca = 10 5. Things to look for: a. Do not reduce subscripts. b. DO NOT CRISS-CROSS!! c. The first word does not need a prefix if there is only one of that element (no mono-) Naming compounds: 1. Write the first nonmetal’s name using the subscript to assign a prefix. If there is no subscript then there is no prefix. 2. Write the second nonmetal’s name always with a prefix and always with ide ending Ex. Ex. dinitrogen hexachloride N2O4 Silicon dioxide SCl6 triphosphorus octabromide SiO Acid: All acid formulas start with hydrogen. All acid names have the word “acid” in the name Memorize the following acids and their formulas: 1. HCl – hydrochloric acid 2. H2SO4 – sulfuric acid 3. HC2H3O2 (CH3COOH) – acetic acid 4. HNO3 – nitric acid Acid: All acid formulas start with hydrogen. All acid names have the word “acid” in the name ex. hydrochloric acid. Again here are the guidelines for writing formulas and naming acids. Writing formulas: 1. Write H as first part of the formula 2. Identify the second part by whether it has hydro in the name or not. Do step 3 or 4!! 3. If hydro is in the name, then write a nonmetal as the second part. 4. If NO hydro is in the name, then use the ending to determine which polyatomic ion to write. a. IC – write polyatomic ion with ate ending b. OUS – write polyatomic ion with ite ending 5. Swap and drop oxidation numbers to identify the subscript on H Ex. Nitric acid Naming Compounds: 1. If the acid is binary (just 2 elements) write: Hydro – nonmetal stem – ic acid 2. If the acid contains a polyatomic ion, change the ending of the ion name. a. ATE – changes to ic b. ITE – changes to ous. c. Then write acid d. NO Hydro in the name!! Ex. H2CO3 Chromic acid HI Hydrochloric acid HC2H3O2