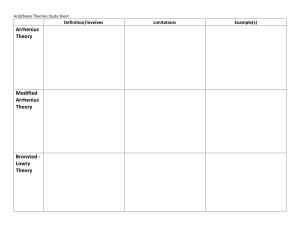
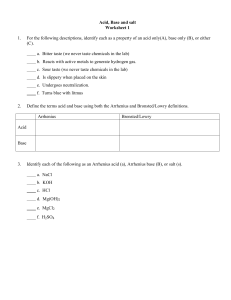
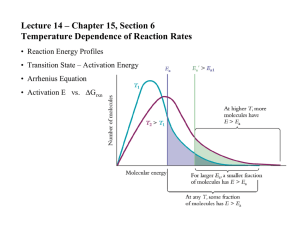
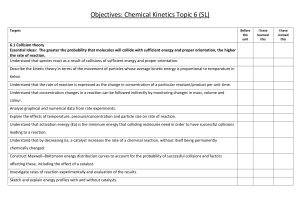
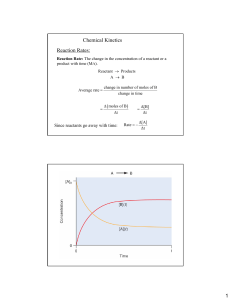
Name: ________________________ Period: ______ Date: ______________ AP Chemistry: Arrhenius Equation Problem YOU DO Problem: Find the Arrhenius equation for the reaction whose data is given in the Arrhenius plot above What is the value of the activation energy? Estimate the k, reaction rate constant, for 700. K. Slope = -14285.71 Y-intercept= 23.1 K=-14285.71(1/t)+23.1 K=-1428.71(1/700)+23.1 K=21.06