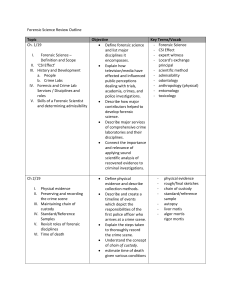
Forensic Science -- Rick Goldstein
advertisement

Forensic Science Lab Manual Rick Goldstein Paideia School 2011-2012 Term 1 Table of Contents Term 1 Labs: Lab #1 Lab #2 Lab #3 Lab #4 Lab #5 Lab #6 Lab #7 Lab #8 Lab #9 Lab #10 Lab #11b Lab #12 Lab #13 Lab #14 Lab #15 Lab #16 Lab #17 Lab #18 Lab #19 Lab #20 Lab #21 Lab #22 Lab #23 Lab #24 Lab #25 Lab #26 Lab #27 Lab #28 Crime Scene Practical #1 – An Introduction Locard’s Exchange Principle Evidence Collection Crime Scene Practical #2 – Surface Recovery Metric System Eyewitness Reliability Measurements in the Lab Glass Density Glass Refractive Index Glass Fracture Patterns Soil analysis Chromatography of Inks Microscopy Fusion of TNT Hair Analysis Fiber Analysis Paint Chip Analysis Toxicology and Drug Identification Arson Investigation Explosives Analysis Fingerprints Ear, Lip and Palm Prints Rope, Key, and Tire Impressions Footwear Impressions and Tool Marks Crime Scene Practical #3 – Collective Analysis Firearms and Ballistics Questioned Documents Polygraphs Forensic Science Name ____________________ Rick Goldstein Lab #1 “In the Beginning, . . . . ” (The First Crime Scene Practical) On this, your first day of Forensic Science, you will be asked to investigate a crime scene to the best of your abilities. You will be divided up, given an area, and asked to do what any professional investigator would be asked to do – collect the evidence and figure out what may have happened. So, you and your group will have a few minutes to plan out your strategy. Then go solve your first crime scene practical. Lab #1 Write up questions: 1. Describe the situation. 2. List what you did and what the rest of the class did. 3. Explain purpose of the lab. Why did we start the year doing this lab? 4. Generally, what did you learn from this experience about yourself and how you function in a group? 5. Specifically, what will you do differently when facing the next crime scene practical? Forensic Science Name ____________________ Rick Goldstein Lab #2 “Glo and Behold” (Locard’s Exchange Principle) Frenchman Edmund Locard is credited with the phrase” Every contact leaves a trace” and is known as the Father of Modern Forensic Science as a tribute to how important this phrase has been to the science of crime solving. You will be asked to analyze what you touch using an alternate light source (ALS). After the initial activity, you and a partner will be given a few minutes to design you own version of this activity to further demonstrate your understanding of Locard’s Principle. Lab #2 Write up questions: 1. List materials and explain procedures of the demo we all did. Include enough detail that someone not present could reproduce the activity. 2. What does Locard’s Exchange Principle mean in every day common language? 3. Clearly connect Locard’s Principle to the class demo activity. Be very specific. 4. List materials and procedures for your own version of the class activity. Go conduct your experiment. Explain your results. 5. How might forensic scientists use this kind of demo in the real world? How practical is this and what kind of results can you expect? Forensic Science Name ____________________ Rick Goldstein Lab #3 “Save me, San Francisco.” (Evidence collection) As you well know from the TV shows and movies you watch, evidence is everywhere at a crime scene. You have done a lab already that addresses how to find evidence. Now, after locating evidence, proper collecting is crucial to make a case. You will want to have Appendix #1 and the relevant sections of the evidence collection chapter from your textbook available to answer some of the following questions. Lab #3 procedure and write up questions: 1. Outline basic evidence collection procedures. What items do you need to collect evidence properly? What steps should be followed to correctly collect evidence. 2. You will be assigned an area of a crime scene to collect certain items from. Go to your area and using the protocol you outlined above, collect your item or items. List two of the items you or your group collected. List proper collection containers and methods or special precautions for each item. Item container method/precautions 1. 2. 3. Pick two items from Appendix #1 that are different from the two listed above. List proper collection containers and methods or special precautions for each item. Item container method/precautions 1. 2. 4. Why does it matter in court that evidence was properly collected? 5. What is the take home message from this lab that you should consider from now through the rest of the course? 6. Your homework is to get a small paper bag and properly collect an item of evidence from an imaginary crime scene in a room in your home. Doesn’t matter what the item is, other than it must be able to fit in the bag, it must be different than anything listed above, and you must be able to describe how to properly collect it. Staple the correctly labeled bag to this lab. a. Item: b. Proper collection method: c. Proper collection container: d. Where item was collected from: e. Likely connection to the crime: f. Date and time collected: g. Sketch scene below: Forensic Science Name ____________________ Rick Goldstein Lab #4 “The Grissom Reality” (CSP #2: surface recovery) This is your second crime scene practical, your second chance to apply what you have learned in class to a mock crime scene. You will be assigned a location and must correctly follow all steps to secure the scene, search for evidence, collect the evidence, and map all collected evidence. Lab #4 Procedure and write up questions: 1. Draw a simple sketch of the crime scene on the back of this lab. Include: a. North arrow, b. Sketch all items found, and c. Draw two fixed points for each item. 2. In a table below: a. List all items collected. b. List distances (feet and inches) to both fixed points for each item, 3. Explain why mapping of evidence is important. 4. How did your group do, compared to “B-4” 5. What can you still do better? Forensic Science Name _______________________ Rick Goldstein Lab #5 “The Power of 10, Two Words: Bo Derek” (The Metric System) The metric system of units is used in most scientific work and this point in your schooling, you should probably know these already. The fundamental unit of linear measurement is the meter,(m). The fundamental unit of mass measurement is the gram (g). The fundamental unit of volume measurement is the liter (l). Commonly used prefixes for the foregoing fundamental units are deci (d) .1 deka- (dk) 10 centi- (c) .01 hecto- (h) 100 milli- (m) .001 kilo- (k) 1000 Thus, 1,000 meters (m) is 1 kilometer (km) .1grams (g) is 1 decigram (dg) .001 liter (L), is 1 milliliter (mL), and so on. Some of the common English units and heir approximate metric equivalents are 1 inch = 2.54 centimeters 1 quart = .94 liters 1 foot= 30.5 centimeters 1.0 mL of water weighs 1.0 g. 1 pound = 453.6 grams 1.0 liter (L) of water weighs 1.0 kg. Factor Label System For Making Conversions A convenient method for converting from one set of units to another without getting mixed up is to use a numerical factor followed by a units label; the factor label method. A pattern that can be followed is What you have x Plus its units Leave a space for factors = What you want plus its units Become familiar with the following two examples. Example 1. A shoe imprint found a scene was found to be 13.0 inches long. How many centimeters long is this shoe? 13.0 inches x 2.45 centimeters = 33.0 cm 1.00 inches Notice that the factor is arranged so that the numerator (top) of one set of units will cancel the denominator (bottom) of another set of units. You use as many factors as you need until the units on the left side of the equation equal the units on the right side of the equation. Example 2. A room is 12.0 ft wide and 15.0 ft long. How many square meters (m2) is the floor in this room? Follow the same pattern, use factors more than once if you need to. 12.0 ft x 15.0 ft x 30.5 cm 1 1 1.00 ft x 30.5 cm x 1.00 m 1.00 ft 100 cm x 1.00 m 100 cm = 16.7 m2 Exercises 1. Fill in the blanks. SHOW YOUR WORK!!! a. 254 centimeter (cm) x = _____________inch (in). b. 120 pound (lb) x = __________kilogram (kg). c. 4 quarts (qt) x = _______________liter (L). d. 400 meters (m) x = _____________yards (yd). e. 6.2 mile (mi) x = _________kilometers (km). f. 1 square inch (in2) x g. 1 cubic decimeter (dm3) x = ______________square centimeters (cm2). = _____________cubic centimeters (cm3). (Hint for #2 - #4: convert all parts of the problem using factor label method to the common unit listed at the answer line, then line up the decimals and add to find the total. So, in #2 convert all units to grams, then add.) 2. Add: 3.4 g, 0.06kg, .67 g, 690 mg, 2dg. = ________________ grams 3. Add 5.2 L, 5300 mL, .44L, 50 mL. = _________________ liters 4. Add: 78 cm, 567 mm, 14 dm, 1.2 m, .023km, 75 mm. = ____________ meters 5. How many liters are contained in a 1.00 cubic meter container and what would it weight if it were filled with water. Ignore the weight of the container. (Hint: 1 L of water weighs 1 kg. Also remember this is a cubic function: L x W x H. Drawing a picture may help you get started.). = _________ liters = _______ kilograms Forensic Science Name ____________________ Rick Goldstein Lab #6 “Say my name, say my name . . . . Wait, what’s my name?” (Eyewitness reliability) Many crimes are witnessed. If the witness makes good observations, has a good memory, can be identified and is interviewed, useful information can sometimes be gathered from the witness statements. Sometimes, that is not the case. There are often reliability issues that could make or break the case. Does it make you wonder, “How observant am I?” Well, we will be finding out. Lab #6 Procedures and write-up questions: 1. Describe both of the memory activities that involved visitors to the class. Describe how well you did. 2. On the back, list the questions I asked in class about you and the classroom and me. After each question, give your original response and the correct response, if your answer wasn’t. 3. Explain the purpose of these memory activities in the context of a crime scene. What does this say about eyewitness identifications that are used as evidence in a courtroom case? 4. How might our legal and judicial systems be flawed in this matter and what can you suggest to do about it? (Give this some thought.) 5. Give an example of a case here in Georgia in the last few years where eyewitness testimony was a crucial issue in the outcome of the case. You may have to look one up. Give the case details. Forensic Science Name __________________ Rick Goldstein Lab #7 “Will you measure up?” (Practicing lab measurements) This is a laboratory exercise intended to help you become more familiar with measurements using the metric system and learning to use a laboratory balance. In this exercise you will determine the density of several objects by different methods. Density is a physical property of matter. It may be used as a means of identification or comparison. The equation for density is Density = mass of object / volume of object. The units for density are g/cm3 or lbs/ft3 Example: Suppose that a man has a body volume of 3 cubic feet and weighs (you can use this for mass) 198lb. What is his density? Would he float in a swimming pool? Density= weight = 198 lb = 66 lbs Volume 3ft3 ft3 Water has a density of 62.4 lb/ft3. Therefore, if the man jumps into a lake or swimming pool, he will sink unless he knows how to swim or to keep himself afloat holding air in his lungs. In other words, an object placed in a fluid sinks if its density is greater than that of the surrounding fluid and floats if its density is less than that of the surrounding fluid. Materials and procedure 1 Balance, electronic Cylindrical Solids (rods) Irregular solid objects Rectangular solids 1 Beaker, 100 mL 1 Graduated cylinder 1 ruler, scissors and string Part A: Density of Rectangular Solids 1. Obtain a rectangular solid from the sample supply. Object # ______________ 2. Measure the three dimensions of the object with a ruler, using the centimeter as the unit of measurement, and record the values below. Length = ________ Width = _________ Height = __________ 3. Determine the volume by applying the formula: V=length x width x height The volume will be in units of cubic centimeters. Volume = _____________ 4.Determine the mass of the object to the nearest .01g by use of the laboratory balance. Mass of the object = ____________ 5. Determine the density of the object by applying the formula D = M/V. Make sure you have the correct units. Density of rectangular solid = _________________ Part B: Densities of Cylindrical Solids 1.Obtain a cylindrical solid from the sample supply. Object letter: _________ 2. Determine its mass to the nearest .01g. Object mass: ________ 3. Since this object is not of the same shape as the rectangular solid, you will have to determine its volume by used of a different mathematical relationship. The formula to be used in this case is: Volume of a cylinder = πr2h Where π = 3.14 r = radius h=height or length of the object 4. Measure the diameter in cm, and divide by 2 to obtain the radius. Diameter = ___________ radius = ____________ 5. Measure the height or length in cm of the object in centimeters. Height = ______________ 6. Determine the volume of the object. Volume of a cylinder = πr2h. Make sure you have the right units. Volume = ______________ 7. Determine the density of the object by using the relationship D=M/V. Density = _______________ Part C: Densities of Irregularly Shaped Solids 1.Obtain an irregularly shaped solid. Object description = ______________ 2. Measure the mass of the object. Object mass = _____________ 3. Since it would be very difficult to obtain the volume of this object by measurement, we will approach this differently. a. Fill a graduated cylinder with water to about one third full. The volume of water is read by noting the position of the bottom of the curve (meniscus) of the liquid level. Starting volume in ml = _________ b. Tie a piece of string around the object. Gently lower the irregular object into the graduated cylinder so that it is entirely below the water level. Note that the water level has risen in the graduated cylinder. Ending volume in ml = ____________ c. Subtract the starting volume from the ending volume for the water level, and you have the volume of the object by a method known as water displacement. Volume of object using water displacement = _________________ 4. You may now find the density of the object by applying the following reasoning. The volume of the object has been indirectly determined and is expressed in milliliters of water, its equivalent volume. A relationship exists in the metric system between units of volume of liquids and units of volume of solids. This relationship is 1mL=1cm3. Thus, all that is necessary to express the volume in milliliter is the volume in cubic centimeters. Volume of the object in cm3 = _______________ 5. Determine the density by applying the equation D=M/V. Density of the irregular object = _____________ Part D. Repeat the steps in Part C (water displacement method) using the rectangular solid sample from Part A (You may need to ask for a very large graduated cylinder) and record the values for the mass, volume and density or this object. Mass = ______________________ Starting Volume = ____________ Ending volume = ___________ Volume of object = ____________ Density = ____________ Lab #7 Write up questions 1. Why were three different methods (Part A, B, and C) needed to measure the densities of different objects? 2. Compare the density of the original rectangular solid object as found in Part A and Part D. Which methods do you think give the most accurate values? Why? a. Density of original rectangular solid object as found in Part A = ________ b. Density of original rectangular solid object as found in Part D = ________ c. Which most accurate and why? 3. Could you have used the method in Part C for determine the volume of any wooden solid object? If so, explain. If not, what would you have to change in the experiment to permit the value for the volume to be more accurately obtained? 4. Could you have used the method in Part C for determine the volume of a solid piece of Styrofoam? If so, explain. If not, what would you have to change in the experiment to permit the value for the volume to be more accurately obtained? 5. Could you have used the method in Part C for determine the volume of a large piece of Sodium Chloride? If so, explain. If not, what would you have to change in the experiment to permit the value for the volume to be more accurately obtained? Forensic Science Name ________________ Rick Goldstein Lab #8 “Stay Glassy, San Diego.” (Density of Glass Fragments) In this experiment, you will determine the density of some glass samples. This is useful forensically to compare two pieces of glass (scene, Q, and suspect, K.) A density measurement might help to determine what type of glass to look for in order to make a comparison. The object of this lab is to determine which glass fragments have similar densities. The density of objects equals its mass divided by its volume (D=M/V). The method for the determination of volume used in this exercise is based on a physics relationship known as Archimedes’s Principle. The principle states that an object immersed in a fluid displaces a volume of fluid equal to its volume. For example, a 1 cm cube of glass placed in water will “push aside” 1 cubic centimeter of water. Another statement of this principle is: “an object immersed in a fluid (water in this instance) is buoyed up by a force equal to the weight of the displaced fluid.” In other words, if we assume that a 1 cm cube of glass weighs about 2.5 g while a 1 cm cube of water weighs 1 g, when the glass cube is placed in water it will weigh 2.5 g minus 1 g, or 1.5 g. Since 1 cubic centimeter of water is equal to 1 g of water, we now have the volume of the glass. Glass from various sources, such as windowpanes, automobile headlights, bottles, and plate glass doors, all have slightly different densities. This makes it possible in some cases to help place a suspect at the scene of the crime if they have broken a glass object and if small fragments have become lodged in their clothing. Forensic labs can analyze glass fragments as small as 1mm wide by 3 or 4 mm long and determine their densities. This exercise will deal with larger pieces of glass. The principles are the same in both cases however, and technique is still very important. Materials and Procedures 1 Balance (+ or – 0.001 g) 1 Beaker, 50 ml 4 Glass samples in a numbered bag (we used bag # _____________) 1 Ruler 1. In the chemistry lab, get a balance and a 100 ml beaker. 2. Push TARE to zero the balance on the scale. 3. Put the first glass fragment on the scale and mass it. Record this value (“weight of glass in air”) to the nearest .001 g on the data table below. 4. Place a 50 mL beaker filled about half way with water, push TARE again, gently place the fragment in the water. 5. Weigh the glass fragment in water. Record this value (“weight of glass in water”) to the nearest .001 g below. 6. Subtract “weight of glass in water” from “weight of glass in air” Convert this value to milliliters. (Recall that 1g of water has a volume of 1 ml.) This is the “volume of glass” measurement in ml. 7. The density of the glass fragment = weight of object in the air (g)/volume of the glass sample (ml) 8. Repeat this process for your other glass fragments. 9. Clean and dry all materials. Return them to the place where you got them. Data Table 1. Weight of the glass fragments in air. Letter _______ weighs_______ Letter _______ weighs_______ Letter _______ weighs_______ Letter _______ weighs_______ 2. Weight of the glass fragments in water. Letter _______ weighs_______ Letter _______ weighs_______ Letter _______ weighs_______ Letter _______ weighs_______ 3. Volume of the glass. (#1 minus #2 and convert to ml) Letter _______ has volume______ Letter ______ has volume______ Letter _______ has volume______ Letter ______ has volume______ 4. Density of the glass fragments (#1 divided by #3) Letter ______ has density ______ Letter _____ has density ______ Letter ______ has density ______ Letter _____ has density ______ Lab #8 Write up questions 1. Based on your density results, which two lettered fragments seem to be a match? 2. How certain (0% - 100%) are you that the fragments you have matched do have a common origin? Give your reasons. 3. Are the densities of glass fragments class or individual characteristics? Explain. 4. Using edge thickness (measured with your ruler) and edge shape, two different kinds of physical matching of glass fragments, which fragments could you exclude as a match(s) for your eventual matched pair? Explain the use of each, including any measurements you took to answer this question. Edge thickness: Edge shape: 5. Are the edge thicknesses and the edge shape of the glass fragments class characteristics or individual characteristics? Explain each. Edge thickness: Edge shape: Forensic Science Name____________________ Rick Goldstein Lab #9: “Becky, What’s My Line?” (Refractive Index (RI) of Glass Fragments) The analysis of glass chips sometimes involves measurements of the refractive index of the glass. Refractive index is a measure of the bending of a ray of light as it passes from air into a solid or liquid. Every material has its own characteristic refractive index. This measurement either shows the possibility common origin of two glass samples, or helps to disprove this possibility. Immersion Methods: When a transparent object such as a glass chip is immersed in a liquid, it is seen by the unaided eye or under a microscope as having a dark or colored boundary, a sort of “halo”. This is called the Becke line. The intensity of this visible boundary around the glass depends on the difference in refractive index between the glass and the liquid. In general, the greater the difference between the refractive index of a specimen and that of a surrounding medium, the more distinct is the Becke line. As the refractive indices of the specimen and liquid approach equality, the Becke line will tend to disappear. Indeed, if the indices of a colorless specimen and the surrounding medium are equal, the specimen will be practically invisible. A difference in refractive index of 0.002 between the glass chip and the immersion liquid can be readily observed. An important advantage of the Becke line is not merely the fact that it indicates a difference between the indices of the glass and liquid, but that it indicates which possesses the higher value. The BECKE LINE MOVES TOWARD the medium of HIGHER refractive index if the focus of the microscope is RAISED and TOWARD the medium of LOWER refractive index if the focus is LOWERED. This observation allows an examiner to properly select a liquid that most closely matches the refractive index of glass. This is shown below: Standard Immersion Liquids: Although the refractive indices of glasses may vary considerably (Table 1), the refractive indices of most glass samples encountered in practice lie between 1.47 and 1.53. Olive oil (1.47), caster oil (1.48) and clove oil (1.54) were used to make the standard solutions for this lab. Table 1: Index of Refraction for Several Glasses Glass Index of Refraction Headlight glass 1.47—1.49 Television glass 1.49—1.51 Window glass 1.51—1.52 Bottles 1.51—1.52 Eyeglass lenses 1.52—1.53 Materials and Procedure: 7 standard solutions of known RI with glass pipette dispenser (purple 1.5430, blue 1.5300, green 1.5175, yellow 1.5050, orange 1.4920, red 1.4820, and brown 1.4667) 1 unknown glass sample in powder form with coffee stirrer as scoop (A-H) (mirror, window, eyeglass, TV, light bulb, jar glass, headlight, or slide) 7 glass slides (one for each known solution) 1 compound microscope a. In pairs, you will be assigned an unknown glass sample. Our Q is letter _______. You will use the Becke lines to determine the RI range of that sample. Each pair will use one dedicated stirrer with their unknown sample, up to 7 glass slides and access to 7 known RI standard solutions. No cover slides are needed. b. Put one drop of the known RI liquid (the colored labeled solutions in brown glass bottles) on a different slide using the capillary tubes. BE VERY CAREFUL NOT TO CROSS CONTAMINATE THE STANDARDS. Using the dedicated stirrer, add a very small amount (one corner of the stirrer is plenty) of the crushed glass from your unknown. Look at the slide under the microscope and find the Becke Line. Determine if it goes out to the liquid or in to the glass fragment. This should help you determine if the known standard solution is of a higher or lower RI than your unknown sample. c. Repeat step b. with higher or lower known standard solutions until you have bracketed the unknown with the smallest range of RI’s that include the unknown. Note which standard solution has a RI that is just above and which is just below your sample, this is your range. My sample range is ________. Lab #9 Write up questions: 1. What is a refractive index (RI)? How is it calculated? 2. Is the RI of a piece of glass a class or an individual characteristic? Explain. 3. Explain the Becke Line and how it is useful. 4. How might determining the RI of a glass sample from a crime scene be helpful to an investigation? Give an example. 5. From the slide of your closest comparison of glass fragment and standard solution, draw the Becke line you see in the microscope and explain whether the line is moving in or out and if that shows that the glass fragment has a higher or lower RI than the standard solution that surrounds it (and say what that standard’s RI is). Forensic Science Rick Goldstein Lab #10 “Pane Pain” Name____________________ (Glass Fracture Patterns) Many crime scenes have glass evidence present. Some of those include high velocity projectiles (usually bullets) impacting the glass. As scientists investigating the scene, it is helpful to be able to tell the direction the bullet was fired from and if more than one bullet is fired, the order that they were fired. Procedure: You will be given sets of bullet holes in different panes of glass. The bullet holes are labeled with letters and the panes are labeled with numbers and “Inside” and “Outside.” Your mission is to figure out the order (first, second, etc.) that each bullet was fired and whether it was fired from inside of the building (moving out) or from outside (moving in). Use the chart below (you should list the first bullet fired (by letter), whether it was fired from inside or outside, then the second bullet fired, and so on). Complete all 4 blue (B1-B4) taped examples, two of the white (W5-W9) window examples and at least one of the truck (T10-T15) windows. Be sure to write the number of the window in the chart below. Pane # Ex: 16 Letter of 1st bullet: in/outside: C In Letter of 2nd bullet: in/outside: A Out Letter of 3rd bullet: in/outside: D Out Letter of 4th bullet: in/outside: B In B1 more here: Lab #10 Write up questions: B2 B3 B4 W_ W_ T_ T_ 1. How can an investigator determine which of two bullet holes in a pane of glass came first? Explain and sketch. 2. How can an investigator determine which direction a bullet came through a pane of glass? Explain and sketch. 3. In the examples you looked at, why is it not always clear the order of bullets in a pane of glass? 4. Headlight glass evidence can be very illuminating in vehicular crime scenes. a. How can one determine if a headlight was on during a car crash? b. Explain the physics of why this works. Sketches are required here. 5. In the spring of 2009, a bullet was found on the floor of an office, 18 inches away from the window’s edge in a pile of glass shards. The window was double paned and had a smaller hole higher up on the exterior pane and a larger hole lower down on the interior pane. The difference in the height of the two holes was 1.75 inches. Based on the evidence, give a reasonable theory of what happened. Forensic Science Rick Goldstein Lab #11b “Down n Dirty” (Soil Lab) Name____________________ Soil can be important evidence at a crime scene because it is easily and inadvertently transferred (remember the Locard’s Exchange Principle). Like other trace evidence, the forensic examination of soil involves comparison of samples in order to establish a link or relationship: the more characteristics that can be matched, the greater the probability of common origin. Low-power microscopy can be used to examine mineralogy, while other characteristics of soil can help to uncover geological processes and geography. Comparative analysis uses physical properties such as density, magnetism, particle size as well as chemical properties, such as pH. Soil is one of the most common materials in the world. The three major components of soil are sand, silt and clay. These major components determine many of a soil’s properties. Sand is the end result of chemical and physical weathering of parent rock. Slow anaerobic decomposition of vegetative matter forms a dark organic soil; such as found in swamps and bogs. The forensic definition of soil includes any artifacts mixed in with the soil, such as fragments of glass, cinders, asphalt, paint, metal, concrete, bricks, etc. as well as natural products. Often, it is the presence of artifacts that make a soil sample unique to a particular location, thereby providing a link to another sample. For example, soil from either side of a galvanized fence usually contains zinc; dirt below an asphalt shingle roof may show shingle stones; potting soils often contain slow-release fertilizer tablets. Materials and procedure: 1 small plastic bag of soil 1 Soil Color guide 1 10ml Graduated cylinder 2 ml dry Calgon 1 pH color test kit 1 Handheld blacklight 1 Forceps 1 Test tube 1 clay triangle 1 crucible and lid 1 Metric ruler Crayons or colored pencils 1 100ml Graduated cylinder Distilled water 1 Hand magnifier 1 Electronic Balance 1 Magnet in a small ziplock bag 1 ring clamp 1 Bunsen burner set up 1 funnel 1. Label the bag of soil with your name, date collected and the specific location. 2. If two soils are from the same location, they should contain particles of similar densities. Remember that density is equal to mass divided by volume. To determine the density of a small amount of your soil you will measure a set volume of soil and then weigh it. Put the 100 ml graduated cylinder on the balance and push TARE. Gently add 30 mL in a graduated cylinder to measure. Do not compact the soil. Mass of 30 ml of your soil: Density of your soil: 3. To determine the relative amounts of sand, silt and clay composition of your soil sample you will find how fast the soil particles settle and the suspension in the water to clear. To the 30 mL of dry soil in the 100 mL graduated cylinder in part 2, add 2 mL of Calgon solution and fill the cylinder with water to the 90mL mark. Cover the cylinder with your hand and shake the graduated cylinder for exactly two minutes to thoroughly mix the sample. Place the cylinder on the table and wait for exactly forty seconds. Measure the volume of material that has settled to the bottom of the cylinder. That is the column of sand that is present in your sample. After thirty minutes have passed measure again. This measurement is to be taken between the upper surface of the sand and the top of the silt. Silt particles are lighter and smaller than sand and so settle out more slowly. Calculate the amount of silt present in your sample. Your sample is to rest overnight (make sure it is on a paper towel with your name on it.) Take the measurement from the top of the silt to the surface of the material that settled overnight, this is the volume of clay in your sample. Add the volumes together. This is the denominator for determining percents. a. mL sand measured: % sand (a./d. x 100) = b. mL silt measured: % silt (b./d. x 100) = c. mL clay measured: % clay (c./d. x 100) = d. total mL measured: 4. Using crayons or colored pencils reproduce as closely as possible the color of your sample here. Color: 5. Compare the color of the soil sample to the color chart. Determine the letter/number combination of the color that best matches your sample. Color: 6. The color of the soil is generally related to the presence of particular minerals or organic matter. Red soils are associated with highly oxidized iron; black soils with organic matter. Wet soil is usually darker than dry soil. What the color of your soil indicates about the content of the sample: 7. Using hand lenses describe the variety of materials found in your sample. This is a list of the variety of materials found in your sample. It is not a list with percents or numbers, but an accurate, detailed list of the variety of things found in your sample. This list might include seeds, sand particles, roots, insects, etc. List contents of your soil: 8. To determine the percent of the soil that is comprised of organic material, you will need to heat the sample in a crucible. First weigh the crucible with the soil sample in it. Then put the sample and crucible on a clay triangle on a ring clamp on a ring stand and heat it with a Bunsen burner. If you are not familiar with this procedure, please ask. a. Mass of the soil sample before adding it to the crucible: b. Mass of the soil sample and crucible before heating: c. Mass of the soil sample and crucible after heating: d. Subtract c. from b. to determine the mass of the organic matter burned off: e. Divide d. by a. and multiply by 100 to get the percent by mass of the organic matter of the soil: 9. Direct ultraviolet light on the soil sample. Note what particles fluoresce, if any. Some minerals, as well as fibers, plastics and paper will also fluoresce. Parts of your sample that fluoresce: 10. Pass a magnet (wrapped in a plastic bag) through the soil to collect and identify any iron that may be present. Do not remove the magnet from the plastic bag. Is there iron present? How much? 11. To determine the pH of your soil sample place a small amount of the sample in a test tube (to the soil line). Add distilled water (to the water line), shake with your thumb over the open end of the tube. Let it settle and then dip the pH paper into the liquid for five seconds. Compare pH paper to the color chart, estimate and record the pH. Estimated pH of your soil: 12. The composition of soil not only determines the color of the soil but also the range of particle sizes (texture). Compare the sizes of your soil particles to the metric ruler. Randomly pick ten particles and measure their diameters (in mm). Add them together and take an average. Ten particle sizes: 1. 2. 3. 4. 5. 7. 8. 9. 10. 6. Average size of 10 particles of soil: 13. Place a quarter-sized amount of soil in your hand. Get it wet and roll the soil between your fingers. Describe the texture and feel. Is it gritty, sticky, slick or spongy? Feel of soil: Lab #11b write up questions 1. Explain which of the above tests would be most helpful forensically. Why? 2. List three of the above tests that yielded a quantitative result a. b. c. 3. List three of the above tests that yielded a qualitative result a. b. c. 4. Based on composition, why might sand evidence not be as useful as soil evidence? 5. Based on the introduction to this lab and your investigation above, define the term “soil” as completely as possible. Forensic Science Name ____________________ Rick Goldstein Lab #12 “Can You Paint with All of the Colors of the Wind?” (Chromatography of Inks) In this lab, chromatography will be demonstrated as a useful laboratory technique for comparing two substances, in this case inks, to either exclude them as a match or say that they are consistent with each other. You will be given three suspect pens and asked to determine which pen(s) wrote a questioned writing. Materials and procedures: 3 known pens (#1, #2, and #3) (K’s) 12 blank filter paper strips 1 pencil 1 jar of methanol developer 1 jar of isopropyl alcohol developer 1 jar of water developer 4 filter paper strips cut from questioned document (Q’s) 1. Get a Ziploc bag with strips of filter paper, pens, and questioned documents. 2. Record your name and either “water”, “alcohol”, or “mix” in pencil at the top of each of three 10 cm length of filter paper. Total 9 strips. 3. Put a dot of each of the three K pens 2 cm from the bottom of each of three of the strips (one from each developer.) 4. Suspend each strip so that the dot is just over the fluid level, but deep enough for the fluid to touch the paper. Keep suspended until liquid is drawn up to the pencil markings near the top. Remove and let dry. 5. Test your Qs, one in each solution. Determine which K (or Ks) were used to make the Q. 6. Verify that you have the correct pen or pens, by using the known (s) to recreate the questioned document. Make a total of three of these. Test one in each of the three developers. Lab #12 Write up questions: 1. Describe the process of chromatography, including how the technique works (not how the lab was set up) and why it works. Illustrations are required. 2. A. Which pen(s) were involved in the questioned writing? ____________ B. Explain how you decided which pen(s) were involved in the crime. 3. Calculate the Rf values (distance substance traveled divided by distance fluid traveled) for one of your Qs and for the K or K’s you think was / were used to make the Q. Show your calculations here. 4. Do the Rf values you calculated support your match? Explain. 5. Do the Rf values have to match exactly to be a match? Why or why not? Staple the 3 original Q strips tested and the 3 filter paper strips you created in step 6 below. Forensic Science Name__________________ Rick Goldstein Lab #13 “Focus here, on me, do it now.” (Microscopes) The microscope is one of the most valuable tools of forensic scientists. It is used to study hair, fibers, seeds, soils, metals, paints, and many other objects. Engravers used glass globes filled with water as magnifying glasses at least 3000 years ago. The simplest microscope is a magnifying glass. Optical microscopes magnify because light rays reflected from an object bend (refract) as they pass through one or more lenses. How big you can make the object depends on the refractive index (bending power) of the glass in the lens. Hand lenses are 3 to 10X. Since the light rays are dispersed out when an object is magnified, the magnified object is not as bright as the original. To make it as bright as it was originally, additional light must be used. This is the purpose of having a mirror under the lens of the microscope. It collects sunlight or light from an auxiliary lamp. The condenser focuses the light collected by the mirror onto the sample. Suppose that you took a small section of a magnified object and place a second lens over it. This magnified section could then be further magnified and we would have a compound microscope. In working with a compound microscope, you should know the following: Working distance - the distance between the specimen and the tip of the objective lens. In general, the higher the magnification, the shorter the working distance. Depth the focus - the thickness of the object that is simultaneously in focus. The higher the power of magnification, the less is the depth of force. Field of View - the area or diameter of the specimen that is in view. The higher the power of magnification, the less is the field of view. Magnification - to determine the magnification of a microscope multiply the magnification of the eyepiece by the magnification of the nosepiece. Materials per pair: 1 Matchbook and matches (in bag) 1 Lens paper 1 Newspaper page 1 Microscope, compound 2 Microscope slides 1 Oregano or tea or other sample Water in a small cup Part A: The Compound Microscope 1 Forceps 1 Pipette 1 Microscope, stereoscopic 2 Cover slides 1 Scissors 1. Get the compound microscope (orange tape on the front) from the cabinet, and carry it (with one hand on the arm and one underneath the base) back to your seat at the table. 2. With a piece of lens paper, lightly wipe any dust and grease from all the exposed glass surfaces. Never use anything else to do this job. 3. Spend the next few minutes becoming familiar with the names and locations of the various important parts of the instrument; figure below will help. The above figure shows the basic parts of a compound microscope. 4. Several important rules are to be noted in connection with the foregoing procedures: To find an object, always start your examination with the low-power objective (red), never with the high, the low-power objective reveals an area of the slide some 20 times greater than the high-power, making it 20 times easier to locate the desired object. To bring the object into focus, always focus upward, with the coarse adjustment. You want to focus upward when your eye is at the ocular (eyepiece). In focusing down, carelessness might crush the specimen or break the slide or lens. When using the high-power objective, never use the coarse (big) adjustment. Compound Microscope Procedure 1. Cut a small sized, lower case letter “e” from a newspaper. Place it as you would read it on a clean slide, and with a medicine dropper, place one drop of water on it. 2. Hold a cover slide at about a 45-degree angle to the slide and then slowly lower it. A gentle tapping will usually remove any bubbles that may be present. Make sure the letter is right side up and straight. 3. Place the slide on the stage and clamp it down. Move the slide so that the letter is in the middle of the hole in the stage. Make certain that the low-power objective is in place. Viewing the stage from the side, use the coarse adjustment wheel to lower the objective until either the stop is reached or the objective is about 2 cm from the cover slide. 4. Turn on the sub-stage illuminator of your microscope. Open up the diaphragm. 5. Now, looking through the ocular, slowly raise the tube with the coarse adjustment knob until the letter is in focus. If you cannot see the object, center the slide more carefully and repeat the whole procedure. The focus maybe made sharper by a slight turn on the fine adjustment knob. 6. The image is in focus 3 to 4 mm above the eyepiece. So, there is no reason to press one’s eye to the ocular. 7. To change to medium-power, make sure that you have focused sharply under low power on the object and centered it in the field. Then carefully swing the medium-power objective into place. The microscopes are parfocal. This means that once the image is brought into sharp focus under low power, it will remain in focus when a different objective is turned into position. The medium-power objective should not strike the slide, though it will come very close. A few turns of the fine adjustment knob, either up or down, should bring the letter into sharp focus. If it does not, go back to step 3 again. Compound Microscope Exercises 1. Under the low power, examine the “e” slide. a. Is the image still right side up? ____________ b. Move the slide to the left: Which way does the image seem to move? ________ c. Sketch what your letter looks like when viewed using this microscope. d. What is the total magnification under low power? _____________ 2. Under medium power, examine your letter. a. Note the many clear spaces within the letter; these are obviously caused by imperfect contact between the press and paper. The higher power lens is able to resolve these imperfections. The microscope then does two things: It enlarges the object and resolves distinctly between closely situated structures in the object (note that magnification and resolution are not the same). b. Take particular note of the fibrous texture of the newspaper. When you focus on different levels by turning the fine focus knob, you will notice that some fibers go out of view and others come in view. This is useful to determine whether a particular object is located above, below, or in the same plane as another object. c. The total magnification of the image formed by the microscope is determined by multiplying the individual magnifications of the ocular and the objective. The magnifying power of these lenses is clearly marked as 10X, 40X, and so on. d. What is the total magnification at the medium power? ___________ 3. Return the compound microscope to the cabinet. Part B: The Stereoscopic Microscope Get a stereoscopic microscope (light green tape on front) from the cabinet, and carefully carry it (with one hand on the arm and one underneath the base) back to your desk or bench. Use the lens paper to clean the lenses. Familiarize yourself with the parts of the microscope. See figure below. Caution: the lamp gets really, really hot. LABEL this Objective Lens Stereo Microscope Procedure 1. Make a second slide with a small lower case letter “a”. Make sure the letter is right side up and straight under the cover slide. 2. Place the new slide onto the microscope base (stage). Turn on the illuminator. 3. Turn the objective lens to the highest power (4X). Look through the right eyepiece and adjust the focusing knobs until the letter is sharp. 4. Turn the objective lens to the lowest power (2X). Without touching the focusing knob, look through the left eyepiece and, using only the left eye, turn the eyepiece, adjusting ring clockwise or counterclockwise until the image is sharp. 5. The adjustment knob allows you to change the power continuously to exactly the best magnification for a given specimen. The stereoscopic microscope allows you to scan an object at a lower power and then to concentrate on some particular detail increasing the power gradually to the desired value. Stereomicroscope Exercises 1. Examine the second slide (the “a”) under the higher stereoscopic microscope setting. a. Is the image right side up? ___________ b. Move the slide to the left: which way does the image seem to move? __ c. Sketch the “a” under the high power. d. What is the total magnification under high power? ____________ e. How does the sketch from this microscope differ from the sketch using the compound microscope? 2. Examine either oregano or tea or some other known under the low power magnification. You do not need to use a cover slide here. a. Sketch either the tea or the oregano or other known under low power. b. What is the total magnification under the low power? __________ 3. Examine the tips of your fingers under the high power of the stereoscopic microscope. Locate the ridges that form a fingerprint. Locate the sweat pores that exist on these ridges. a. What is the total magnification under the high power? ___________ b. Below, sketch part of the ridges and sweat pores of your left forefinger. 4. From time to time a forensic laboratory may be asked to see whether a tornout paper match comes from a partially used book, usually taken from an accused person. Matchbooks contain two pieces of cardboard secured in the book with a staple. The individual match body is formed by a series of partial cuts in this cardboard: thus each layer of matches was originally a single piece of cardboard. The obvious first attempt to match a torn-out match to a partially filled matchbook requires physically fitting the torn edges of the match to the corresponding portion of the torn book. If that does not work, a forensic examiner will then try to compare the suspect match with matches remaining in the book in order to establish an adjacent relationship. The most significant features to look for in the comparison of paper matches are: overall color, width and thickness; contents of the reprocessed cardboard, including colored fibrous material, and aluminum foil; and the presence of continuous fibers between adjacent matches. PLEASE DO NOT REMOVE ANY MATCHES FROM ANY MATCHBOOKS IN THIS LAB. a. Match bag # ______________ b. Sketch the fiber patterns on your two matches when looked at on edge (the thin way.) Match A Match B c. The match (pick either A or B) that matches the matchbook is _____________ d. What did you base your decision on? Explain. Forensic Science Name ____________________ Rick Goldstein Lab #14 “Wow, the Pretty Colors” (Fusion of TNT) Finding crime scene trace evidence is often easy to do, as there is often so much of it. Figuring out what to do with it can be more difficult. Different light wavelengths and different lenses can show characteristics and properties of the component compounds and elements. In this lab you will look at several chemical samples under polarizing lenses. This can be used not to make an identification, but to show comparable samples or to eliminate inconsistent samples. Materials and procedure: 3 Slides of different samples 1 Compound microscope 2 Polarizing lenses 1 alcohol burner matches colored pencils / crayons 1. Place the TNT slide on the compound microscope at 10X. Focus it. Increase the magnification to 40X. Below, sketch the TNT slide at 40X, without any polarizing lenses. 2. Add one polarizing lens on light source and describe what the TNT slide looks like under a compound microscope, 40X, with one polarizing lens on light source. 3. Add a second polarizing lens by holding it slightly above the stage or platform. The first lens should still be sitting on the light source. Sketch the TNT slide under a compound microscope, 40X, with two polarizing lenses, one on light source and one above stage. (Use colored pencils or crayons.) 4. Now light your alcohol lamp with the match. 5. This is where team efforts will be needed. One partner will need to be heating the chemical on the slide, while the other will be ready to rotate the filter between the lens and the stage and be ready to observe through the microscope. 6. CAREFULLY, while holding the side of the slide with the label on it, wave the center of the slide above the flame, moving it constantly. If you over heat the slide, it will shatter in your hand. Once it is melted, quickly place the slide on the microscope and observe. If the TNT has solidified before you can observe it, melt it again carefully and view again. 7. If time permits, try the same procedures with the other chemicals on slides. You should see VERY different looking results. Lab #14 write up questions: 1. Explain what technically (chemically) happens when you melt the TNT on the alcohol burner and watch it cool under the microscope. 2. Why did the TNT look different when you added a second filter? Make sure you explain what the second polarizing filter does. 3. Why would every substance (TNT, DDT, and any other chemical compound) have a unique look as it melts and solidifies? 4. How might what you have seen in this lab be useful forensically? 5. I am always looking for good descriptors of what you are seeing through the microscope in this lab. How would you best describe what you saw? Forensic Science Name ___________________ Rick Goldstein Lab #15 “Hair Thee Well” (Microscopic Examination of Hair) Hair is a very common form of evidence in many cases of homicide, sexual assault and burglary. Hair evidence can link a suspect to the scene of the crime, indicate the entrance or exit route of the criminal, show contact with the victim, or serve to identify clothes or shoes of the suspect. Hair from any part of the body exhibits a range of characteristics, such as color, length, and diameter. Even hair from different parts of the same area, the crown, sides, and rear of the head, for example, will differ somewhat. It is, therefore, necessary for the forensic examiner to keep this in mind when collecting reference hairs and to obtain an adequate supply to compare with the suspect's hair. Usually, the collection of several dozen hairs from relevant parts of the body will suffice. The parts of a hair that are easily seen by use of a microscope under magnification are the medulla, the cortex, and the cuticle as shown in Figure 1. Many animal hairs are easily distinguished from human hairs by the size and shape of their medullae and the patterns of their cuticle or scale structure. Synthetic fibers have no medulla or scale pattern and are therefore readily distinguishable from animal hair. Figure 2 shows several scale patterns. This lab is a qualitative exercise to determine characteristics of hair samples. Figure 1: Structure of hair Materials: 3 Microscope slides – glass 3 Cover slides - glass Clear nail polish (for scales) Glycerin or water (for wet mounts) Part A. Human Hair characteristics Figure 2: Scale patterns of hair 1 Compound microscope Microscope tissue paper 1 Forceps Hair samples (human and animal) 1. Pull a strand of your head hair and place it on a glass microscopic slide. 2. Place a drop or two of glycerin or water on the hair in order to hold it in place, and put a cover slip over the hair. This is known as a wet mount. 3. Place the slide on the stage of the compound microscope, and adjust the magnification to 100x. (Red lens) 4. Locate the root end of the hair, if it has one. If the hair has been forcibly pulled out, you will likely see a bulb-shaped enlargement. This is the hair root, and adhering to it, you will see small pieces of flesh and tissues, which surround the hair root. 5. Make a sketch of the root end of your hair. 6. Is the medulla fragmental (that is, present in isolated spots), interrupted (long columns with open spaces now and then), or continuous (unbroken column)? Is it entirely absent? At higher magnifications, some hairs may show irregularly shaped air spaces, known as cortical fuzi, dispersed throughout the cortex. 7. Make a sketch of the medulla you observe. 8. Describe the color, relative diameter, and pigment distribution of the hair. Go look at other students to see the relative diameter of your hair. 9. Examine the tip (external end) of the hair. This end can be determined by the gradual tapering of the hair. If the hair had been cut recently, you will see a square appearance at the end. If hair has split ends it is normally due to artificial waving, bleaching, although repeated brushing may also produce this effect. (See power point slides.) 10. Sketch the external end of your hair. Part B: Animal Hair Characteristics Animal: ____________ 11. Make a sketch of the root end and sketch the external end of the animal hair. Root External end 12. Describe and sketch the medulla of the animal hair. Part C: Scale Patterns Scale patterns are of little value in human hair comparisons but can aid in distinguishing animal hairs from crime scenes. The pattern of cuticle scale is useful in determining the species origin of hair. In human hair, the scales overlap smoothly, whereas in other mammalian species they protrude in a rough, serrated form. It is difficult to examine the scales directly, so what is most often done is to prepare a cast of scales. Follow these instructions: 1. Smear a glass slide with a thin layer of clear nail polish. 2. Before the clear nail polish dries, which takes place very quickly, place a strand of animal hair on the surface of the polish. 3. Before the polish has thoroughly dried, but after the surface becomes partially solidified (a few seconds), lift the strand of hair off the slide. You should now see an imprint of the hair in the polish. 4. Place the slide on the microscope’s stage, focus, and observe the scale pattern of the imprint. 5. Now try observing the scale pattern on a strand of hair placed on a slide. Which is more easily seen? 6. Make a sketch of the scale pattern impression. Part D Slide Collection 1. Examine the human hair slides (red dots) from the slide collection. Match both Qs to Ks. Explain why you think they match. Sketch all Qs. Q _____ = K _______________, reason for match: Sketch: Q _____ = K _______________, reason for match: Sketch: 2. Examine the animal hair slides (green dots) from the slide collection. Match all Qs to Ks. Explain why you think they match. Sketch all Qs. Q _____ = K _______________, reason for match: Sketch: Q _____ = K _______________, reason for match: Sketch: Q _____ = K _______________, reason for match: Sketch: Forensic Science Name ____________________ Rick Goldstein Lab #16 “Don’t Thread on Me” (Fiber evidence) Fibers may become important evidence in incidents that involve personal contact, like homicides, assaults, or sexual offenses. Cross-transfers may occur between the clothing of suspect and victim. The force of impact between a hitand-run victim and a vehicle often leaves fibers, threads, or even whole pieces of clothing adhering to parts of the vehicle. Fibers may also become fixed in screens or glass broken in the course of a breaking-and-entering attempt. Regardless of where and under what conditions the fibers were found, their usefulness depends on whether their origin can be reduced to a limited number of sources or a single source. Given the mass production of garments and fabrics, it is hard to find a fiber with individual characteristics. Early in the twentieth century, the first manufactured fiber, rayon, was made. It was followed in the 1920s by the introduction of cellulose acetate. Since the late 1930s, scientists have produced dozens of new fibers. In fact, the development of fibers, fabrics, finishes, and other textile-processing techniques has made greater advances since 1900 than in the five thousand years of recorded history before the twentieth century. Today, such varied items as clothing, carpeting, drapes, wigs, and even artificial turf attest to the predominant role that manufactured fibers have come to play in our culture and environment. This lab will investigate several ways to analyze fiber evidence. Part 1 Flame tests Materials and Procedure: 1 Tea candle 1 Forceps 1 Scissors 1 packet of matches 1 Bag of fibers [with aluminum foil in it] 1. Get one bag of unknown fibers 2. Look at the numbered known fibers in the folder & decide which three most closely visually match the crime scene letter assigned to you. 3. Cut off a very small piece [5x5 mm – about half the size of a pencil eraser] of fiber from each of your suspect samples. 4. Fold the piece of aluminum into a bowl-like shape 5. Place the tea candle on the aluminum. 6. Light the candle. 7. Holding the first known fiber in forceps, bring it close to, but not touching, the flame. 8. Record your observations in the data table below: does it ignite, melt, or curl? 9. Holding the fiber in forceps, touch the fiber to a flame. 10. Record your observations in the data table below: does it ignite quickly or slowly? Does it sputter, drip, or melt? 11. Remove the fiber from the flame and describe how it behaves. Does it self extinguish, continue to burn, or continue to glow? 12. Observe any odor associated with the fiber in the flame. 13. Repeat steps 7 - 12 for the other two of the closest known fibers. 14. Then repeat steps 7 - 12 for the crime scene unknown. 15. Once you have melted and burned all the unknown pieces of fiber, blow out your tea candle. And, clean up. Data table for Lab #16. Fibers [ Suspect # ____ Approaching flame In flame Removed from flame Odor Suspect # ____ Suspect # ____ Crime scene letter ____ Lab #16 write up questions: 1. Based on the information from your data table above, which known matches with the crime scene unknown? 2. What was the strongest evidence you used to make the match? 3. Does fiber evidence exhibit individual and/or class characteristics? Explain. 4. Explain why it is unlikely to find two indistinguishable colored fibers coming from randomly selected sources (see Text p. 230) 5. Explain the difference between manmade & natural fibers. Give two examples of each. a. Difference: b. 2 examples of man made fibers: c. 2 examples of natural fibers: Part 2 Microscopic examination of fiber evidence Examine the textile fiber slides (yellow dots) from the slide collection and identify the three unknowns assigned to you. Sketch each and explain how you visually determined the matches. a. Unknown (Q) ____ is a match with the known (K) slide _______________. Reason: Sketch: b. Unknown (Q) ____ is a match with the known (K) slide _______________. Reason: Sketch: c. Unknown (Q) ____ is a match with the known (K) slide _______________. Reason: Sketch: Forensic Science Name ____________________ Rick Goldstein Lab #17 “Chip off the Old Block” (Paint chip comparisons) Paint chips are common trace evidence at many crime scenes. Thy can be matched by layers, shape, and chemical composition. In this lab, you will be looking at the microscopic layering of sample chips of automobile paint, both for how paint is applied as well as how it can be used as evidence. Materials and procedures: Various automobile paint chips in prepared wax slices Stereomicroscope 1. You will be given a set of wax slices each with a small chip of automobile paint that has been cut in cross section. BE CAREFUL NOT TO OVERHEAT these wax samples, if you do, the sample will be of no further use to anyone. 2. View each slice IN THE ORIGINAL CONTAINER under the lowest magnification of the stereomicroscope. Lab #17 write up questions: 1. On the back, sketch a cross section of each of the known (K) reference samples and the one unknown (Q) sample. Use colored pencils. 2. Using words only, without additional sketches present your evidence of how you determined the identity of the unknown paint chip. ID the Q. 3. Explain why paint chips can be useful forensically. Are individual and/or class characteristics both demonstrated in paint chips? Explain. 4. Explain how paint generally is applied to a surface (see text p 232). 5. Explain how automobile paint particularly is applied (see text p 234). Be sure to explain the four organic coatings. Forensic Science Name ____________________ Rick Goldstein Lab #18 “Your Love is My Drug” (Toxicology/ Drug Identification/Alcohol Testing) Following the lecture and having read the two text chapters, you should be able to answer some basic questions related to toxicology, drug identification and implied consent/alcohol testing. Lab #18 Write up questions: 1. List the 5 categories of drugs in the US Controlled Substances Act (it is OK to look them up the in text.) a. b. c. d. e. 2. Give an example of each. a. b. c. d. e. 3. Explain how the five categories are different from each other by comparing the addictiveness and the medical use of each. a. b. c. d. e. 4. Explain how the fields of toxicology and drug identification are different. 5. What samples (kinds of evidence) does each area work with? 6. 7. 8. 9. What is the legal limits for alcohol in blood and breath for teens? _________ What is the legal limits for alcohol in blood and breath for adults? _________ Why are there different levels in the above two questions? Pick one of the drug testing labs from class. a. Describe how the test worked. b. List the set number you worked with. ______________ c. Describe the results you got (color change, etc.) d. What does the result you got mean? 10. What are the two basic types of breath alcohol tests? (hint: one is a preliminary screening test.) 11. Describe the steps in both types of Alcohol Breath Tests and what a result might look like for each. a. Preliminary screening test steps: Results: b. __________________ (other test) steps: Results: 12. Give a quick overview of the Tylenol Poisoning Case, including dates, places, number of victims, type of poison, suspects, where is the case now, etc. Forensic Science Name ____________________ Rick Goldstein Lab #19 “Fire Burning, Fire Burning” (Arson Investigation) In fire scene investigation, there is often more evidence than can be collected and processed in a reasonable time. Burned material, smoke, and water are everywhere and in some structures upper levels have collapsed onto lower levels, complicating the investigation even more. Investigators need to be able to locate the most likely spots to collect debris. In this lab, you will be trying to understand the way structures burn (and collapse) so you can determine where the likely Point of Origin is and center your investigation there. To start with you and a partner will be building a standard structure along with all of the other pairs. After completed, you will be given an accelerant and a location to pour it. You will light the accelerant and burn down the structure. Once all groups are done, you and your partner will be assigned a charred structure to investigate. You may refer to your lecture notes, the text or internet references. Your job is to locate the likely Point of Origin and explain why you have reached that conclusion. Materials and Procedures: 90 Popsicle sticks Elmer’s glue Accelerant in jar aluminum tray sand matches compass paper pencil 1. Using all 90 Popsicle sticks and the Elmer’s glue, construct a structure that uses all of the sticks and only the glue to hold them together. No tape or any other type of glue is allowed. 2. Fill the aluminum tray 1 inch deep with sand. 3. Place the completed structure on the sand. 4. Sketch your structure and at the top of the page draw an “N” for north and write the number of your structure. a. Number of your structure: _____________________ 5. Once the class is in the garage and you have been assigned a location, orient your tray with your sketch so that the north arrow you drew agrees with the compass (including the angle of declination – we will go over how to do this in class.) 6. Next, you will be given a location to pour your accelerant (either NW, NE, SE, or SW). Pour all of the fluid on the appropriate corner of the structure and immediately light it. a. Corner assigned to your structure _________________ b. Accelerant used _______________________ 7. Mark your Point of Origin on your sketch with an X. 8. Stay with the structure until it has burned completely. 9. You will be investigating all of the other groups’ scenes, sketching your results, and determining the Point of Origin. Lab #19 write up questions: 1. Explain the basics of arson investigation. What would indicate a high likelihood of arson, not accidental fire? 2. How is the Point of Origin determined? 3. Look over your sketch of the house you built and burned. Is the Point of Origin clear? Is N drawn on? Is the number clearly visible? 4. On the back, sketch the remains of each of other the houses that you are investigating with the number of the house at the north side (and the top of your drawing, and a N arrow) and your best guess as to the Point of Origin marked with an 0. 5. Justify your choice of Point of Origin in all of the other houses, using your powers of observation and what you have learned in this unit of the course. 6. You will be given a GC/MS print out from an actual case (attach it to this lab). Using any written or electronic resources, determine the most likely accelerant used in the arson. Include a copy of your research (attach it also to this lab) and how you made your determination. a. Unknown number: _________________. b. Best guess for accelerant used: _____________________ c. Reasoning: Forensic Science Name ____________________ Rick Goldstein Lab #20 “This Place About To Blow” – (Explosives Analysis) Following the lecture and having read the text chapter, you should be able to answer some basic questions related to explosives and explosives analysis. Lab #20 Write up questions 1. What are the names of the two basic types of explosives? a. Give an example of each. b. Explain how you can tell which is which. 2. Pick one of the three explosives demonstrated in this unit in the chem. lab. a. Which one of the two types in question #1 it is and how do you know? b. Explain what happened when it was demonstrated. Correct vocabulary (especially verbs) is important here. 3. What object was destroyed for our class by ATF? a. Give three reasons why you think it was blown up and not dropped from a great height or shot by a bullet. i. One: ii. Two: iii. Three: 4. In reconstructing the object in question #3, a. How could an investigator determine the exact make and model number of the object? b. What was the exact make and model number? c. Attach a picture of the object, cut out and glued/taped here. d. Find an exact address within 10 miles of our school where you could buy one of these. List the address here. 5. In the video of the ATF Post Blast Explosives School, two large truck tires were used in a demo. a. What were the two explosives used in the demo? b. How were the two types of explosives used differently? Forensic Science Rick Goldstein Lab #21 “Around the Whorl” (Fingerprints) Name _____________________ Fingerprints are a very common form of physical evidence. It requires considerable expertise to be able to accurately classify prints and match prints with each other. If a suspect’s fingerprints match those found at a crime scene, this is highly conclusive proof of link between the two. In this experiment you will investigate the methods used in developing and lifting latent fingerprints from a number of objects, made of a variety of materials. You will also try to match the prints with inked prints. Latent prints are those invisible prints left on an object by a person. These must be developed using dusting powders or chemical solutions. Inked prints are those taken directly from a person’s fingers with an inkpad or block. Plastic Prints are those in a soft substance (like clay), where the print is imbedded in the substance. The origin of the use of fingerprints is lost in history, although it is known that the Chinese used fingerprints thousands of years ago. In 1886, a Scottish physician, Henry Fauld, first published the view that fingerprints could be used for identifying individuals. We owe the beginnings of our present system to Sir Francis Galton in the 1880s. Sir Edward Richard Henry developed a simplified system for classifying fingerprints, which was adopted by Scotland Yard in 1901. There are a number of basic fingerprint patterns (arches, loops, whorls). The fingers on a person’s hand may contain a number of patterns. These patterns are shown in Figure 1. You should know the characteristic appearance of each of the patterns for this exercise. Figure 1 Basic Fingerprint types: #3, #4, #6, and #7 should be radial or ulnar loops depending on the side. The tips of a person’s fingers have small friction ridges on them. Along these ridges are small pores that secrete salt (NaCl), water, and proteins. It is those substances, along with oil that may be picked up by touching the hairy portions of the body, which will be deposited on objects that come in contact with the surface of our fingers. There are a variety of fingerprint dusting powders. The choice of powder color depends to a large extent on the color of the object being investigated for prints. We will make use of three colors of dusting powder: white for use on darkcolored objects and black for use on light-colored objects, and fluorescent for multicolored objects. Latent prints developed by powders and lifted from the object by use of transparent tape and hinged lifters. Lifters are available with black, white, or transparent to the adhesive surface of the tape. The tape is placed on a paper whose color will provide a suitable contrast with the print. The transparent tape also provides an immediate positive print. You will use both transparent tape and hinge lifters in this experiment. The hinged lifter consists of a plastic, adhesive-backed sheet attached to a colored paperboard. When the examiner is ready to lift the print, a lifter is selected. (This will be determined by the color of the powder used.) The backing is removed from the plastic sheet, exposing the adhesive. The plastic sheet is now pressed against the developed print, allowing the print to be picked off of the surface. The plastic sheet is then pressed against the colored paperboard. You may not be able to obtain really clear latent-print development in this exercise, and perhaps this will serve to illustrate to you the importance of technique in this operation. Practice is very important. You will develop a few of your own latent prints in the beginning of this exercise. If you should find that your skin is quite dry and does not deposit prints very well, this can be remedied. Handling paper dries the skin very quickly. If this is the case, rub your fingers along the side of your nose to pick up some skin oil, or run your fingers through your hair, which will accomplish the same purpose. Good prints will usually result if this is done. Materials: Fingerprint kits with powders and dusters Glass beaker Hand magnifying lens (10x or stronger) Lifters, hinged: black, white, transparent Scissors UV lamp Procedures for lifting fingerprints Metallic powder and applicator Dark-colored ceramic bowl Soda can Perfect Print ink pads Transparent tape Fingerprint cards, FBI type 1. Use your left index finger, a glass beaker (non-porous) and Perfect Print pad to leave a print. Use transparent tape to lift print from the beaker. Label with your name and “left index on glass”. Tape print to the space below. Wipe off beaker. 2. On a dark part of a ceramic bowl, place “oiled” left thumbprint. Dust with silver powder, lift with clear tape and place on black construction paper, or use a black hinge lifter. Label print with your name and “left thumb on dish”. Tape it below. Wipe off dish. 3. On a colored portion of a soda can, place your right index fingerprint. Dust with fluorescent powder and lift with white hinge lifter. Label with your name and “right index on can.” Tape hinge lifter in the space below. Wipe off can. 4. On a beaker, put your oiled right thumbprint. Using the metallic powder, the special metallic brush, and a clear hinge lifter, remove the print. Label with your name and “right thumb on beaker.” Tape hinge lifter in the space below. Wipe off beaker. 5. Using the Perfect print pads, ink and print the middle of all 10 fingers through the first joint, outsides of thumbs, four fingers together, and knife-edges of both palms on the FBI card on the next page. Label with your name. Identify each of your 10 fingerprints either as a loop, whorl, or arch on the FBI card. Turn in this FBI card to Rick. 6. Look at the known right thumbprints on the plastic slides labeled with the owner’s initials; these are the Ks. Match the ENTIRE group of numbered Qs, also on plastic slides. Below, list all of the Ks alphabetically. Then match each to the correct Q. You may work as a group of two or three. Lab #21 Write up questions: 1. In procedure #5 above, you printed your fingers. List below how many loops, whorls and arches you had on each hand. 2. How does the frequency of your prints match with the average in the US? 3. Do fingerprints show individual and/or class characteristics? Explain. 4. In procedures #1 and #4 above, you took prints off of the same surface, how do the two look different? 5. What do you see when you look at all 4 of the prints you lifted under the fluorescent light?? Forensic Science Name ____________________ Rick Goldstein Lab #22 “Hear Me, Kiss Me, Slap Me” – (Ear, Lip, and Palm Prints) Although fingerprints get most of the print attention on TV and in movies, there are many other types of prints that can be used to identify or eliminate a person. In this lab we will be looking at three: ear (safe crackers of old would listen to the tumblers in a safe and leave their ear prints), lip (straws, drinking glasses and cans are all good sources of lip prints), and palm prints (often found fully or in part at scenes with fingerprints.) It is important to consider the differences between individual and class characteristics when looking for and at these prints. Materials and procedures: 3 x 5 index cards black fingerprint powder lipstick paint 2 poster boards for the class glue stick 4 x 6 index cards black fingerprint powder brush soda can paint brush wide clear tape 1. Ear Print: Remove any earrings on your right ear. Rub with oil from your face. Press ear to 3 x 5 index card. Dust the ear print using a fingerprint brush, a light swirling circular motion and a very small amount of black carbon dusting powder. Carefully tape over the print to secure it. Label your ear print with your name on the same side of the card as the ear print. Use a glue stick to add it to the class “Ear Print” poster. 2. Make a second right ear print as you did above and attach it to the lab below. Circle three individual characteristics on your print. 3. Explain why the three, circled individual characteristics above are not class characteristics of your ear print. 1. 2. 3. 4. Lip Print (cheiloscopy): Put on lipstick; get help if this is new for you. Fold a 3 x 5 index card along the 1/3 – 2/3 line (1/3 on the top and 2/3 on the bottom) and make a lip print with both lips on that fold. Watch this demonstrated. Do a second print of your lips on the bottom (in the 2/3 part) of the same side of the index card by pressing your lips flat on the card. Write your name on the card. Add your labeled card to the class “Lip Print” poster. 5. Make a second lip print and attach it below. Circle three individual characteristics on your lip prints. 6. Explain why the three, circled individual characteristics above are not class characteristics of your lip print. 1. 2. 3. 7. Palm print: Tape a 5 x 7 index card around a soda can. Cover your left palm with a THIN layer of paint. Roll your hand over the can. Remove paper. Label and set in lab to dry; you will attach this below when dry. 8. Do a second left palm print on a second paper, but this time without the can, just flat on a table. Label this and let dry. Add this to the class “Palm Print” poster. 9. Comparing your two palm prints, they should appear slightly different. Explain why. Which might be more useful forensically? Explain. 10. How are the ear, lip, and palm prints different from the rope, key and tire marks from the previous lab? Explain. Forensic Science Name ____________________ Rick Goldstein Lab #23 “Mark My Words . . .” – (Impressions: Rope, Key and Tire Marks) Rope impressions are often found in kidnapping, rape, murder and other crime scenes. Key marks and tire marks are also found in a wide range of crime scenes. In this lab, we will be looking at premade impressions and trying to match them to known ropes, keys and tires. We will also be making impressions and casts from the molds, common techniques that are vital to viable evidence. Materials: Bag of Rope samples (K’s) Rope impressions (Q’s) Key molds (Q’s) Plaster of Paris Tire Newspaper Colored Clay Key sets (K’s) Water Ziploc bag Paint Procedures and Write up questions for Lab #23: 1. Rope Impression: Roll a small ball of clay into a 5-inch long cylinder “snake” shape. Press all Ks (numbered 1-7) in your bag into the clay to make seven impressions. Compare the assigned crime scene Qs (labeled with a letter between A and P) to the seven Ks (numbered). Sketch and identify all of your Qs with the correct K. Q Sketch matching K 2. Key comparisons: Compare all of your assigned clay key molds (Qs are labeled with numbers) with the five suspects (K’s are stamped with letters). Clearly identify each of the five matched pairs. Matched pairs are: 3. Use one of the keys you carry or any key from this lab for this question, but everyone must have a different key for this and the next question. List three class characteristics and three individual characteristics of any one of the keys. Which key used? ___________________ Class Characteristics 1. Individual Characteristics 1. 2. 2. 3. 3. 4. Key Cast: Flatten a 3 cm ball of clay to 1.5 cm thick circle in a plastic dish, not on the lab bench or desk. Press the key used in the above question into the clay until it is flush with the clay surface. Carefully remove the key. Cast the key mold with Plaster of Paris (using the technique from lab #22.) You don’t need much plaster. Make one batch for the class. Let harden overnight, carefully remove cast, label with name or initials. Each person needs his or her own cast. Put your cast in the container in the back of the class. 5. Tire Print: Each person should find or make an individual mark on a tire. Not using too much paint, “ink” the individualized portion of your tire and roll it firmly over newspaper to practice then over a piece of computer paper with your name on it. Clean up your mess. Each person should have his or her own page. Staple your page to the back of this lab. Again, clean up your mess. 6. After the paint dries, circle and label your individual characteristic on the computer paper. 7. Tire Print comparison: Using the set of painted tire prints or plaster casts of tire marks assigned to you (your Qs) determine which K made each. In the two columns below, list the matches. My Q is: The K that made it was: 8. Which of the techniques in this lab are the hardest to do well? Why is that? Forensic Science Name ____________________ Rick Goldstein Lab #24 “Stomp and Circumstance” -- (Footwear impressions and Tool marks) Commonly left behind at crime scenes, footwear impressions and tool marks can be valuable links between the crime scene and a suspect. Following the correct collection protocol can make the difference between a usable impression and one that is worthless. In this lab, we will focus on examining, sketching, recovering and preserving footwear impressions and tool marks. Materials: Stereomicroscope Screwdriver impression (Q’s) Biofoam Plaster of Paris Ziploc bags Colored clay Screwdrivers (K’s) Shoes (K’s) Mixing containers Water Procedures and Write up questions for Lab #22: 1. Using a stereomicroscope on 4X, examine in detail the crime scene tool impression (SD #1-8) assigned to you. Sketch your impression below. My impressions is SD #________. 2. List three class characteristics and three individual characteristics of your impression in #1 above. Class characteristics Individual characteristics 1. 1. 2. 2. 3. 3. 3. Examine the known screwdrivers (#1-8). Which is a match for the tool marks impression assigned to you? _________________ Explain your answer. 4. Take your assigned K shoe (S #1-30) and carefully make a footwear impression in the Biofoam. Watch the technique demonstrated before attempting your own Biofoam impression. 5. List three class characteristics and three individual characteristics of your footwear impression. Class characteristics Individual characteristics 1. 1. 2. 2. 3. 3. 6. Sketch the impression in the Biofoam in the space below (not to scale). 7. Circle the three individual characteristics on your sketch above. . 8. Make a cast of your shoe Biofoam mold using Plaster of Paris. Mix a small amount of plaster (2.5 parts plaster to 1 part water) and water in a Ziploc baggie. Mix quickly (20-30 seconds is all you have) and then pour it into the impression. (Watch this demonstrated – pour from a low altitude and not directly on the delicate impression). Add screen. Add a top plaster layer over the screen if needed. Leave overnight. Label your box and cast with the shoe number and your name. Do not wash any plaster down the sink. Clean up. Forensic Science Name ______________________ Rick Goldstein Lab #25 – “We’re All in This Together” – (Crime Scene Practical #3) As a class, you will be looking for and analyzing the evidence listed below. Each of you will be responsible for one area of evidence on your own. Ks are provided for you. For this lab you will file a Final Report for your portion of the investigation. Type this. Make it official looking; include your analysis and conclusions. Back up your professional opinions with a detailed list of evidence that you analyzed, including: Item #, what it was match to, and describe how match was made. For some types of evidence, sketches are a really good idea. Feel free to ask for your own set of the FBI cards for your classmates. (Dazzle me with the practical knowledge and skills you have picked up so far in the course. Appropriate use of vocabulary is a must. If anyone assisted you, say what exactly he / she did to help you. If you assisted anyone, say what you did.) Types of evidence 1. Wayne Williams Wannabe: fiber matches – examine the item of clothing provided for fibers. Match the Q to the carpet Ks. Say where the clothing item has been. 2. Singing the Blues: trace evidence – Match Qs (see blue dot slides to be) with Ks provided in slide boxes. (2 people could work on this, each on her or his own set of slides.) 3. Life’s A Beach: sand evidence – Match Qs (which may be a combination of two or more different samples) with Ks provided. Say which place or places the sand was from. 4. Bad Deal: toxicology and drug ID evidence – look at the results of the tests performed on the victim, the suspect and the powders. Figure out what happened in the case. (challenging) 5. Burn’in Down the House: arson evidence – Locate the point of origin of the fire from the photos. And look at the GC patterns to determine the accelerants used. Verify your sources. 6. Step’in Out: footwear evidence – Match Qs in clay with Ks provided as casts. 7. Chips Ahoy: automobile paint chip evidence – Match Qs and Ks provided. 8. Kiss Me: lip print evidence – Match Qs on spoons with Ks on poster. List 5 basic types of lip prints and the correct name for the study of lip prints. 9. Where the Rubber Meets the Road: tire evidence – Match partials Qs to tire Ks. 10. The Future: plastic left middle finger prints – match 3d impression Qs to FBI card Ks. 11. Hear Me Roar: left ear print evidence – match Qs on window with Ks on poster. 12. All Thumbs: left thumb prints on ceramic bowl – lift and match to plastic slides. 13. Sticky Wicket: left little finger prints – Match Qs on shelf supports to Ks on FBI print cards. 14. Palm-wonderful: left palm prints-- Match Qs on glasses with Ks on FBI print cards. How you will be evaluated: You will be evaluated on your written work (including any drawings), on your ability to properly analyze the evidence, on the results of your analysis, and on your understanding of the underlying science as presented in your written work. Although it is a class project, your individual grade will also reflect your level of demonstrated commitment to the group effort (by what you did, and how you helped the other investigators.) Forensic Science Name ____________________ Rick Goldstein Lab #26 “Kill Shot” – (Firearms and Ballistics) The field of Firearms and Ballistics analysis requires good pattern identification skills, lots of basic physics, and a heaping dose of patience. This is detailed forensic science at its finest. For centuries, firearms craftsmen knew that the rifling grooves cut into the barrels of guns imparted unique properties to each weapon. It wasn’t until the 1880’s that medical and forensic professionals began to use this information in crime solving. In this lab, we will be looking at how bullet matching is done. Materials and procedures: 2 magnifying glasses Comparison microscope (shared by class) set of bullets You will be given a set of four pairs of bullets that have been fired already. The known bullets (Ks) are small manila envelopes that are labeled with the info on the type of bullet, the weapon that fired it and the id number of the sample. The questioned bullets (Q’s) are in small plastic vials with the id numbers on them. Your job is to match them. CAUTION: be very careful to not mix up the bullets when comparing them. ALWAYS KEEP the K’s on the RIGHT (“You know the right thing to do”) and the Q’s on the Left. If you get stuck, you can use the big comparison microscope in the back of the class, but have it explained to you before using it. Write up questions for Lab #26: 1. List the matches below. Set # ____________ a. b. c. d. 2. Sketch one of the bullets from each of the four matches. a. b. c. d. 3. Describe how to a match a Q bullet to a K bullet. What do you look for? (hint: what does 6L mean?) 4. How can you eliminate any two bullets as a match? 5. Describe what IBIS is and how it is used in forensics. 6. How is IBIS different and similar to the activity you did in this lab? 7. Describe what GSR is and how it is useful in forensics. 8. What are some of the limitations of using GSR as an investigative technique? 9. Diagram and label an unfired bullet in cross section, the long way. Include the following terms: cartridge, powder, bullet, and one more of your choosing. 10. Diagram and label a handgun in cross section, the long way. Include the following terms: magazine, breech face, trigger, ejector, and one more of your choosing. 11. Describe something related to bullets, ballistics or firearms from one of the firearms web sites: firesarmsid.com or firearmstechnology.com that no one else in the class will choose. Forensic Science Name ____________________ Rick Goldstein Lab #27 “Can’t Touch This” – (Questioned Documents) Questioned Documents (QD) is a large field that includes all written, drawn, printed and computer generated documents. This includes checks, contracts, wills, money, ransom notes, works of art, and many others. Some forgeries are easier to detect, like the permission note your friend gave the 3 rd grade teacher signed “John’s mom.” Others like some top US currency forgeries or master art forgeries are nearly impossible to detect and take highly specialized training. In this lab, we will focus on handwriting as a way to investigate the subtleties in solving QD cases. Materials and procedures and write up questions: 3 3 x 5 note cards 1 Blue ink ballpoint pen 1. On three 3x5 note cards, copy the “writing” on the board in red ink; do not sign two, but do sign one. It is OK to use your normal handwriting, it need not be cursive, but you must write the same way for all three. Hand to Rick. 2. Write the phrase “The quick brown fox jumped over lazy dogs.” in your same normal handwriting below. Then sign it with your name in cursive. 3. Describe 10 characteristics of your K handwriting sample in question #2. a. b. c. d. e. f. g. h. i. j. 4. Have each classmate write the same sentence from question #2 below and sign it. 5. Looking at each of your classmate’s K samples in question #4, describe one unique identifying characteristic for each above. 6. Next, you will be presented with a bag of shredded note cards. Using the Ks you have above for the members of your class, determine who wrote the Q words presented to you on the cut up cards. List whose writing you have here and explain using the Ks how you arrived at your conclusion: 7. Give an example of an individual characteristic in handwriting. 8. Give an example of a class characteristic in handwriting. 9. What could you do to disguise your handwriting? Explain and show what you mean. 10. How could investigators still find that the writing you disguised above was actually made by you? Forensic Science Name ____________________ Rick Goldstein Lab #28 “I Love The Way You Lie” – (Polygraph) Polygraphs are the machines that we commonly refer to as lie detectors. They measure some of the physical changes that occur in response to questions and their answers. Polygraphs have been around for many years, some more effective than others. In police circles, there are several references to a mythical case (immortalized in several movies and books) where detectives put an aluminum foil covered colander (sieve) on a suspect’s head and ran some random wires to the back of a Xerox machine. Previously, three pages had been placed in the machine a head of time that said “Truth,” “Truth,” and “LIE.” So the police asked two easy questions that the subject would get right and the third question was “Did he do the crime?” The suspect was overwhelmed by the superior technology, caught in a lie, confessed, and a myth was born. Obviously there is a lot more real, hard science that goes into actual polygraphs, as our visiting expert will demonstrate. Following the demonstration, you should be able to answer these questions completely and confidently. Don’t make me bring out the colander. . . . 1. Are polygraphs reliable AND usable in court? Explain. 2. Sketch & describe the polygraph set up, include all items on or under subject. Clearly label each 3. What are the three main measurements that a polygraph monitors? 4. Draw and label the parts of a sample print out. 5. What must happen in the mind of a subject for a polygraph to work? a. Did this happen in our demo? b. Explain. c. Did this happen in the heart rate lab? d. Explain. 6. List your heart rates for the following activities for 60 seconds: 1. Laying down: 2. Sitting: 3. Standing: 4. Doing sit-ups: 5. Running up the stairs: 6. Listening to uncomfortable music: 7. Listening to relaxing music: 7. What surprised you most about your results in the above question?