CHEMISTRY SUBTERM 1 STUDY GUIDE
advertisement

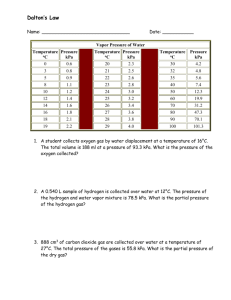
CHEMISTRY SUBTERM 1 STUDY GUIDE 1) The diameter of a UFO is 387,000,000 m. What is this number expressed in scientific notation? 2) How many significant figures are there in the measurement 810,300 grams? 3) What is the measurement 3003 L rounded off to three significant figures? 4) What is the temperature -49 C expressed in Kelvin? What is “absolute zero?” 5) How many protons, electrons and neutrons does an atom with an atomic number 65 and a mass number 140 contain? 6) All atoms of the same element have the same _____. 7) In which of the following is the number of neutrons correctly represented? 8) Which of the following sets of symbols represents isotopes of the same element? 9) Of the elements Pt, Sc, V, Li and Kr, which is a nonmetal? 10) Which of the following categories includes the majority of the elements? 11) Who first arranged the elements according to atomic mass and noticed a periodic recurrence of their physical and chemical properties? 12) What is the next atomic orbital in the series 1s, 2s, 2p, 3s, 3p, 4s? 13) What is the electron configuration of calcium? 14) How many valence electrons are there in an atom of nitrogen? 15) How many electrons does sulfur have to gain in order to achieve a noble-gas electron configuration? 16) What is the charge of a particle having 16 protons and 15 electrons? 17) How many unpaired electrons are there in a phosphorus atom? 18) Which of the following occurs in an ionic bond? 19) What is the shape of the water molecule? 20) Which atom in a water molecule has the greatest electronegativity? 21) In which of the following groups of ions are the charges all shown correctly? 22) Which of the following correctly provides the names and formulas of polyatomic ions? 23) Which set of chemical name and chemical formula for the same compound is correct? 24) Which set of chemical name and chemical formula for the same compound is correct? 25) What is the correct formula for hydrocyanic acid? 26) How many moles of silver are there in 3.8 x 1024 atoms of silver? 27) How many ammonium ions are there in 3.2 mol of (NH4)2S? 28) What is the molar mass of magnesium phosphate? 29) Which of the following elements exists as a diatomic molecule? 30) The chemical formula of aspirin is C9H8O4. What is the mass of 13.0 moles of aspirin? 31) If the density of an unknown gas Z is 1.25 g/L at STP, what is the molar mass of gas Z? 32) What is the empirical formula of a compound that is 40% sulfur and 60% oxygen by weight? 33) The ratio of carbon atoms to hydrogen atoms in a molecule is 3 to 7. What is the molecular formula of this substance if its molar mass is 86.0 g? 34) All of the following are empirical formulas EXCEPT ____. 35) Which expression represents the percent by mass of nitrogen in N2O4? 36) What are the missing coefficients for this skeleton equation? C6H6 + O2 CO2 + H2O 37) Aluminum chloride and bubbles of hydrogen gas are produced when metallic aluminum is placed in hydrochloric acid. What is the balanced equation for this reaction? 38) Write a balanced equation to represent the decomposition of aluminum oxide? 39) The equation MgOH + 2HCl MgCl2 + H2O is an example of which type of reaction? 40) In order for the reaction Zn + 2HCl ZnCl2 + H2 to occur, which of the following must be true? 41) Which of these is an INCORRECT interpretation of this balanced equation? 2S(s) + 3O 2(g) 2SO3(g) 42) In the reaction 2CO(g) + O2(g) CO2(g), what is the ratio of moles of carbon monoxide used to moles of oxygen gas used? 43) How many liters of oxygen are required to react completely with 7.2 liters of hydrogen to form water? 44) How many moles of aluminum are needed to react completely with 1.2 mol of FeO? 2Al(s) + 3FeO(s) 3Fe(s) + Al2O3(s) 45) Iron (III) oxide is formed when iron combines with oxygen in the air. How many grams of Fe 2O3 are formed when 1.3 g of Fe reacts completely with oxygen? 4Fe(s) + 3O2(g) 2Fe2O3(s) 46) Identify the limiting reagent and the volume of product formed when 88 L CS2 reacts with 64 L O2 to produce CO2 gas and SO2 gas. CS2(g) + 3O2(g) CO2(g) + SO2(g) 47) Lead nitrate can be decomposed by heating in an environment of excess oxygen. What is the percent yield of the decomposition reaction if 5.6 g Pb(NO3)2 is heated to give 1.2 g of PbO? 2Pb(NO3)2(s) 2PbO(s) + 4NO2(g) + O2(g) 48) Which of the following is NOT a true statement concerning limiting and excess reagents? 49) If the volume of a container of gas is reduced, what will happen to the pressure inside the container? 50) What happens to the temperature of a gas when it is compressed? 51) What happens to the pressure of a gas inside a container if the temperature of the gas decreases? 52) The volume of a gas is doubled while the temperature is held constant. How does the gas pressure change? 53) The volume of a gas is reduced from 4 L to 2 L while the temp. is constant. How does the gas pressure change? 54) A gas occupies a volume of 2.4 L at 14.1 kPa. What volume will the gas occupy at 3.9 kPa? 55) A sample of gas occupies 2.3 mL at –34 C. What volume does the sample occupy at 52 C? 56) If a balloon containing 3 L of gas at 30 C and 75 kPa rises to an altitude where pressure is 45.7 kPa and temp is 12C, the volume of the balloon under these new conditions would be calculated as follows 57) A breathing mixture used by deep-sea divers contains helium, oxygen, and carbon dioxide. What is the partial pressure of oxygen at 101.4 kPa if PHe= 82.5 kPa and PCO2 = 0.4 kPa? 58) When a container is filled with 3.00 moles of H2, 2.00 moles of O2, and 1.00 mole of N2, the pressure in the container is 768 kPa. What is the partial pressure of O2? 59) If the volume of a container of air is reduced by one-half, what happens to the partial pressure of oxygen within the container? 60) Which of the following gases is the best choice for inflating a balloon that must remain inflated for a long period of time?