BOHR-RUTHERFORD DIAGRAMS
advertisement

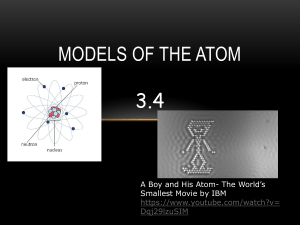
Name: ______________________________ BOHR-RUTHERFORD DIAGRAMS In order to draw you must know: 1) The atomic number gives you the # of protons and electrons. 2) The atomic mass - atomic number gives you the # of neutrons. To Draw: 1) Draw a small circle for nucleus. Inside write the number of protons and neutrons. Include the symbols P+ and NO beside the numbers. 2) Draw a concentric circle outside the nucleus to represent the electron orbits. The number of circles is determined by the amount of electrons an element has. 3) Draw dots on these circles starting with the closest circle to the nucleus. This Orbit can only hold 2 electrons but each orbit after can hold 8. Only begin a new orbit when the previous has been filled. ( 2-8-8) Draw a Bohr-Rutherford Diagram for: Lithium Oxygen Aluminum