lecture
advertisement

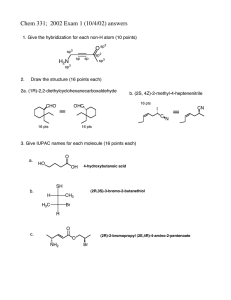
Lecture Manuscript For Thursday January 20tthh, 2000 Umbreen Mir 971299900 CHM 331S Professor M. Denk January 27th, 2000 1,1-Dilithio-organyls 1,1-dilithio-organyls can be obtained by hydroborating alkynes with 9-borabicylcononane in a two-step synthesis reaction: R CH C 9 -BBN Li BBN n-BuLi C R R CH CH2 BBN CH Li Elimination Reactions of LiHal in Lithiumorganyls The reactions of 1,2 and 1,3-dichloroethane with lithium do not produce 1,2 and 1,3dilithio-organyls, but instead are ideal procedures for synthesizing anhydrous metal chlorides: Cl Li H2C CH2 + LiCl Cl Li Cl Cl + LiCl This is the general method in obtaining metal halides in the anhydrous form (in aprotic solvents such as ether, hexane and toluene). This method works well with electropositive metals of group 1-3, such as Mg, Ca and Fe with the exception of inert elements, beryllium and boron. Anhydrous metal chlorides are important synthetic starting materials, in particular for organometallic reactions. Samarium (II) iodide is a synthetically important reducing agent which generates radicals from organohalides. It is prepared from 1,2-diiodo-ethane and samarium, and is generally better if it is prepared fresh before it is used. 1,4-dilithium compounds can be obtained, but the procedures are very tricky. Most of this 1,n-dilithium chemistry was developed by a Dutch chemist named Bickelhaupt: Hal Li Hal Li 1,2-Dehydrobenzene 1,2-dehydrobenzene is a relatively unstable compound. It can be synthesized from 1,2-dihalo compounds, or the standard method in which an intermediate diazonium salt is formed: 1,2-Dehydrobenzene Hal Li Hal -N2 + -CO2 NH3 COO- iC5H11-ONO N2 + COO- Dehydrobenzene has been stabilized in the form of metal complexes, particularly Ta, by the work of an Australian chemist named Bennet. Dehydrobenzene chemistry also produces unusual hydrocarbons such as biphenylene and triptycene. The synthesis of triptycene is a high yielding reaction, if done properly, 7080% product can be obtained: anthracene Elimination Reactions of LiH in Lithiumorganyls The lithiation of a hydrocarbon, leads to the elimination of LiH. The addition of GeCl21,4-dioxane results in a ring closure forming 1,3-diaza-germole, and unsaturated germole: tBu NH NH tBu n-BuLi THF NLi NLi GeCl2.(Dioxane) tBu tBu N N Ge - two LiCl THF + Ge N N tBu tBu tBu tBu tBu -LiH N Li Ge N tBu An older example of a reaction that eliminates LiH, is the nucleophilic alkylation of pyridine with RLi which leads to a 1,2-addition: R-Li N H N Li R -LiH N R In 1865, the first alkaloid with high toxicity was synthesized: N Chichibabin Reaction The Chichibabin reaction is widely used today, mainly to make 2-amino substituted pyridines, via the elimination of NaH: NaNH2 N H N -NaH NH2 N NH2 Na The same reaction cannot be carried out with OH- because it is not a strong enough nucleophile to break the pyridine ring. Instead, N-methyl-pyridinium salts are used, and then OH- is added: Stability of Organolithium Compounds PhLi, n-BuLi, sec-BuLi, tert-BuLi, and methyl lithium are commercially available organolithium compounds. The most commonly used, n-BuLi is produced on a large scale, for example, 17,000 litres are available. Purity of Organolithium Compounds Organolithium compounds are sold as stock solutions : 1.6 molar, 2.0 molar, 10.0 molar. The 10.0 molar solutions are pyraphoric, and must be handled with care. Determining the contents of organolithium solution is difficult because they decompose in air and moisture. Organolithium compounds with a -hydrogen, eliminate LiH to form alkenes: Impurities of Organolithium Compounds R R Li Li OH2 O2 R H R Li Heat H C 2 H + LiOH + LiH OLi CH2 The decomposition products: LiOH, R-OLi and LiH cannot be distinguished from RLi through simple titrations with an acid because the sum of these impurities will be determined. Therefore, a technique, known as the Gilman double Titration is an alternative in determining only the contents of RLi. Gilman Double Titration The Gilman Double Titration, established in the 1940’s, determines the contents of organolithium compounds (RLi). Since RLi is the only compound that reacts with dibromoethane, it is easily removed from other impurities. This process involves two titrations, in which the difference between 1. and 2. is the content of RLi: 1. Conventional Titration LiH LiOH 2. Titration of Impurities LiH Dibromoethane LiOH R-Li R-Li R-OLi R-OLi + R-Br + Li-Br + alkene Today, the Gilman Double Titration can be done by optical indicators, which involves a two-step deprotonation reaction. Reduction of Hydrocarbons Lithium reacts with hydrocarbons to give radical anions: -. Li + These radical anions display strong colours (naphthalene=greenish, anthracene=blue), and are air and moisture sensitive. Anthracene and naphthalene are not used as nucleophiles, but instead are strong reducing agents and are used as singular electron transfer catalysts. Arene Radical Anions Chromium hexa-carbonyl is a colourless white solid with stable carbonyls. Since the radical anions of naphthalene and anthracene are very strong reducing agents, they are ideal for reducing chromium hexa-carbonyl to chromium penta-carbonyl. THF is used to solvate the lithium cation: CO CO 2 CO CO CO Cr CO CO Anthracene OC Li, THF OC - + Cr CO CO CO A traditional way to synthesize the same product involves using liquid ammonia and lithium. The following reduction can be conducted by both (a) Na and C10H10 as well as (b) K and small amounts of naphthalene: (a) R R R N C S N Na, C10H10 N R (b) N 2X C: -Na2S R N C C N N N R R R R R N N molten K C N S C: Anthracene N + K2S R R Formation of –ate Complexes An undesirable side reaction with organometallic compounds is the formation of –ate compounds. An example is illustrated by the formation of a borate complex: Ph BF3.Et2O Ph-Li Li + B Ph Ph Ph Only small traces of BPh3 are produced because boron is a strong Lewis acid, which can easily react with an equivalent of PhLi. The problematic formation of –ate complexes can be avoided by using Grignard reagents: BF3.Et2O Ph Ph-MgBr Ph B Ph Use of Tetraphenylborate Ph4B- forms insoluble salts with large cations. Therefore, tetraphenylborate is ideal for distinguishing Li and Na, which have water-soluble salts, from the alkali cations K, Cs and Rb, which have water insoluble salts. Grignard Reagents Grignard reagents were important because they were the most accessible organometallic compounds. They can be obtained by mixing dry ether and organic halides, preferably bromides because they are more reactive, and because Cl has a lower boiling point: R Et2O X R MgX If nothing happens during the reaction, the best way to activate Mg is through sonification. Grignard Reagents in Solution Grignard reagents exist as monomers and dimers: R OR2 -Et2O R Mg R2O Mg X Et2O R2O R X Mg X OR2 Strong donors (TMEDA>ET3N>THF>ET2O) and dilution work well with monomers, whereas weak donors and high concentrations suit dimers. Synthesis of R2Mg One implication of the following equilibrium is how it is possible for two R substituents to end up on one Mg. A general synthesis of R2Mg is from R-MgX with 1,4-dioxane, which results in the formation of a precipitate of MgX2: O R-MgX + R-Mg-R + MgX2.(1,4-dioxane) O Formation Mechanism The reaction of organohalides with Mg begins with a single electron transfer step, (SET reaction) which involves radical intermediates: (1) (2) (3) (4) weakening of R-X bond dissociation of radical anion to a radical + an anion formation of R-Mg bond extract using Et2O R-MgX(Et2O)2 R-X ¯• R-X Mg (1) Mg R• + X¯ (2) Mg R + X¯ (3) Mg (4) Arthur Ashe (III) proved the intermediacy of radicals by trapping it with suitable substrates: X CH2 . MgX CH2 . MgX The first dicoordinate organomagnesium compound was characterized by Eaborn in 1989: SiMe3 SiMe3 Me3Si C Mg SiMe3 C SiMe3 SiMe3 References Denk, M. (1999) Lecture notes on Inorganic Chemistry II: Organometallics.