CHM 254 Organic Lab Quiz 1 A Name____________________
advertisement
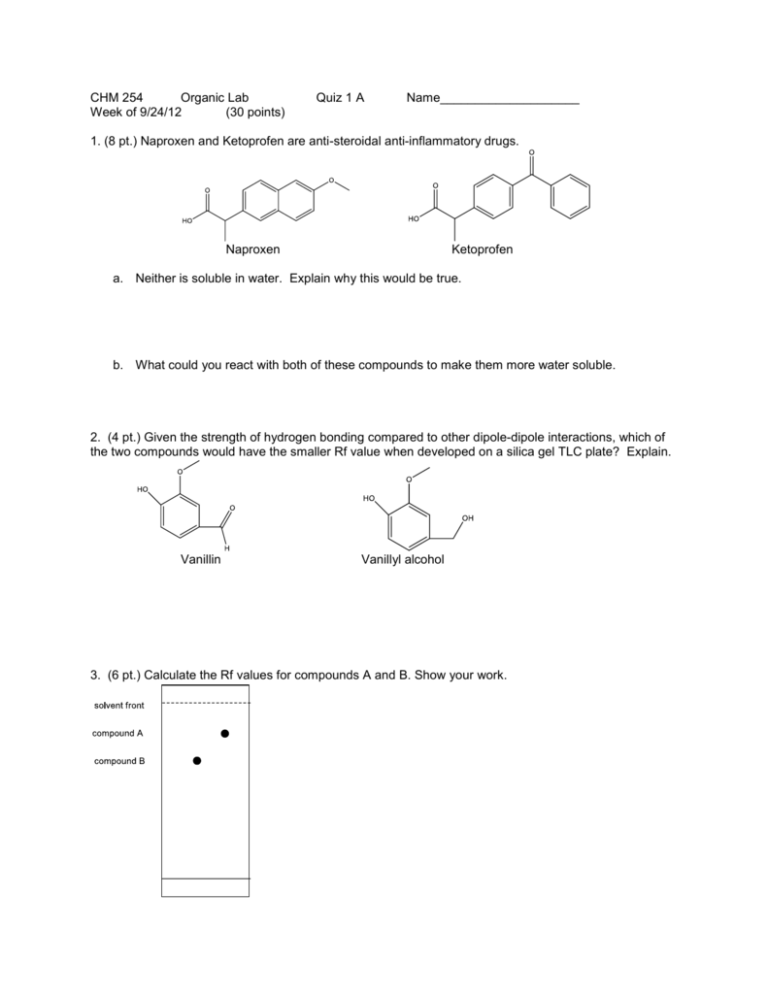
CHM 254 Organic Lab Week of 9/24/12 (30 points) Quiz 1 A Name____________________ 1. (8 pt.) Naproxen and Ketoprofen are anti-steroidal anti-inflammatory drugs. Naproxen Ketoprofen a. Neither is soluble in water. Explain why this would be true. b. What could you react with both of these compounds to make them more water soluble. 2. (4 pt.) Given the strength of hydrogen bonding compared to other dipole-dipole interactions, which of the two compounds would have the smaller Rf value when developed on a silica gel TLC plate? Explain. Vanillin Vanillyl alcohol 3. (6 pt.) Calculate the Rf values for compounds A and B. Show your work. 4. (5 pt.) What two methods are used to visualize colorless non-fluorescent compounds on a TLC plate? 5. (7 pt.) Over time some organic compounds react with air (known as air oxidation). If this occurs for a bottle originally containing a pure compound, what will happen to its melting point over time?











