Graphing Atmospheric Data
advertisement
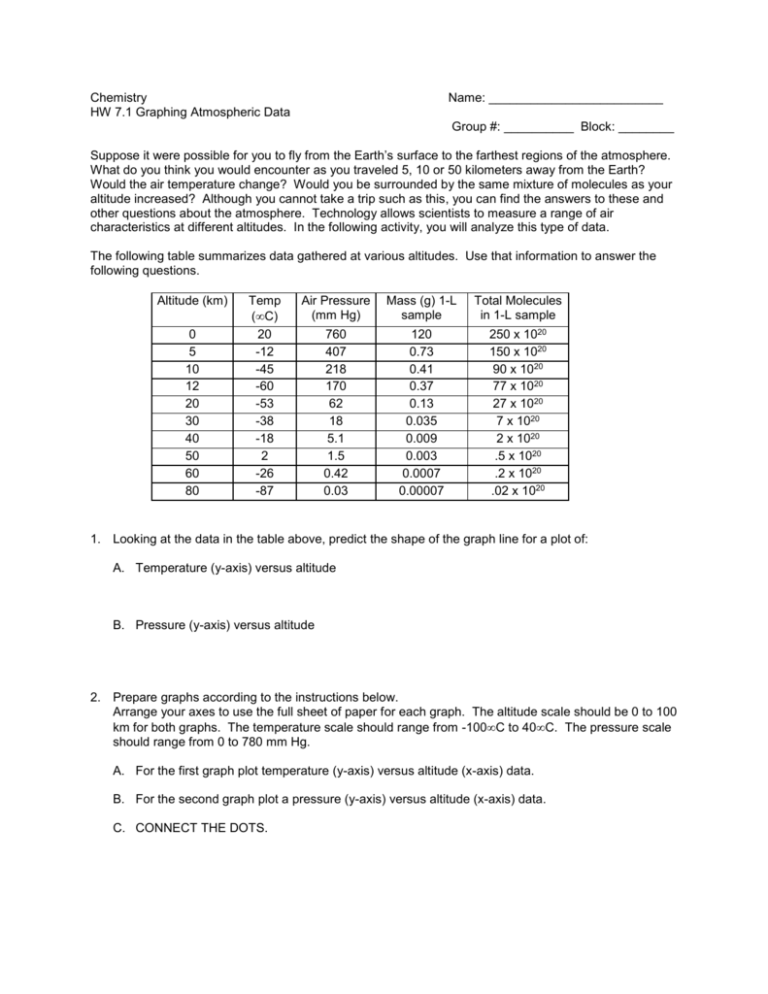
Chemistry HW 7.1 Graphing Atmospheric Data Name: _________________________ Group #: __________ Block: ________ Suppose it were possible for you to fly from the Earth’s surface to the farthest regions of the atmosphere. What do you think you would encounter as you traveled 5, 10 or 50 kilometers away from the Earth? Would the air temperature change? Would you be surrounded by the same mixture of molecules as your altitude increased? Although you cannot take a trip such as this, you can find the answers to these and other questions about the atmosphere. Technology allows scientists to measure a range of air characteristics at different altitudes. In the following activity, you will analyze this type of data. The following table summarizes data gathered at various altitudes. Use that information to answer the following questions. Altitude (km) 0 5 10 12 20 30 40 50 60 80 Temp (C) 20 -12 -45 -60 -53 -38 -18 2 -26 -87 Air Pressure (mm Hg) Mass (g) 1-L sample Total Molecules in 1-L sample 760 407 218 170 62 18 5.1 1.5 0.42 0.03 120 0.73 0.41 0.37 0.13 0.035 0.009 0.003 0.0007 0.00007 250 x 1020 150 x 1020 90 x 1020 77 x 1020 27 x 1020 7 x 1020 2 x 1020 .5 x 1020 .2 x 1020 .02 x 1020 1. Looking at the data in the table above, predict the shape of the graph line for a plot of: A. Temperature (y-axis) versus altitude B. Pressure (y-axis) versus altitude 2. Prepare graphs according to the instructions below. Arrange your axes to use the full sheet of paper for each graph. The altitude scale should be 0 to 100 km for both graphs. The temperature scale should range from -100C to 40C. The pressure scale should range from 0 to 780 mm Hg. A. For the first graph plot temperature (y-axis) versus altitude (x-axis) data. B. For the second graph plot a pressure (y-axis) versus altitude (x-axis) data. C. CONNECT THE DOTS. 3. Compare the ways in which air temperature and air pressure change with increasing altitude. A. Which follows a more regular pattern? B. Explain this behavior. 4. Based on the graphed data, would you expect air pressure to rise or fall if you traveled from sea level (0 km) to: A. Pike’s Peak (4301m above sea level)? ____________ B. Death Valley (86 m below sea level)? ____________ 5. Suppose you took one-liter samples of air at several altitudes. How would the following change? Hint look at the data provided in the data table. A. Mass of air sample B. Number of molecules 6. Scientists often characterize the atmosphere as having four layers: troposphere (nearest Earth), stratosphere, and the mesosphere. Mark the graph from Question 2 which you think can be used to show the different layers. Indicate these layers with lines at appropriate altitudes to indicate where you think the boundaries between the layers might be.











