Chapter 4 and 5
advertisement

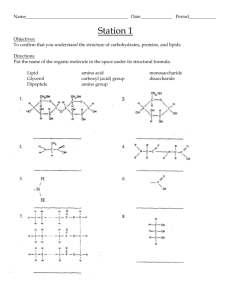
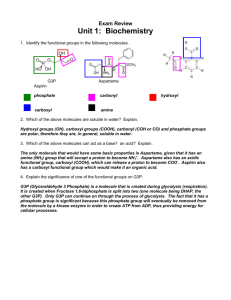
CH. 4 & 5 STUDY GUIDE: ORGANIC CHEMISTRY KEY TERMS organic chemistry secondary structure carbon dehydration synthesis(=condensation reaction) hydrocarbon maltose keratins isomer sucrose pleated sheet single bond lactose tertiary structure double bond hydrolysis quaternary structure functional group polysaccharide denatured proteins hydroxyl starch conjugated proteins carbonyl glycogen nucleic acids aldehyde cellulose DNA ketone chitin parts of a nucleic acid amino fatty acid purines sulfhydryl saturated adenine carboxyl unsaturated guanine phosphate phospholipid pyrimidines macromolecule steroid cytosine monomer amino acid thymine polymer peptide bond uracil monosaccharide polypeptide RNA glucose disulfide bond disaccharide primary structure fibrous proteins alpha helix 1 WORD ROOTS hydro - = water (hydrocarbon: an organic molecule consisting only of carbon and hydrogen( iso - = equal (isomer: one of several organic compounds with the same molecular formula but different structures and, therefore, different properties) enanti - = opposite (enantiomer: molecules that are mirror images of each other) carb - = coal (carboxyl group: a functional group present in organic acids, consisting of a carbon atom double-bonded to an oxygen atom) sulf - = sulfur (sulfhydryl group: a functional group that consists of a sulfur atom bonded to an atom of hydrogen) thio - = sulfur (thiol: organic compounds containg sulfhydryl groups) con - = together (condensation reaction: a reaction in which two molecules become covalently bonded to each other through the loss of a small molecule, usually water) di - = two (disaccharide: two monosaccharides joined together) glyco - = sweet (glycogen: a polysaccharide sugar used to store energy in animals) hydro - = water; - lyse = break (hydrolysis: breaking chemical bonds by adding water) macro - = large (macromolecule: a large molecule) meros - = part (polymer: a chain made from smaller organic molecules) mono - = single; - sacchar = sugar (monosaccharide: simplest type of sugar) poly - = many (polysaccharide: many monosaccharides joined together) tri - = three (triacylglycerol: three fatty acids linked to one glycerol molecule) QUESTIONS 1. What is the role of carbon in the molecular diversity of life? Why is carbon SO important? 2. Describe the structure of a typical monosaccharide such as glucose. Write out a condensation reaction between two glucose molecules, and explain hydrolysis. 2 3. Fill in the following table on the functional groups: Functional Group Molecular Formula Names & Characteristics of Organic Compounds Containing Functional Group -OH Aldehyde or ketone; polar group Carboxyl -NH2 Thiols; cross-links stabilize protein structure Phosphate 4. Explain the difference between a saturated and an unsaturated fatty acid. Explain how three fatty acids can react with glycerol to make a fat. 5. Diagram a phospholipid molecule and point out the polar and nonpolar ends. Identify the hydrophobic and hydrophilic ends of this molecule. 6. Identify the alpha-carbon, the carboxyl group, the amino group and the R group of an amino acid. 7. Differentiate between the various levels of protein structure-primary, secondary, tertiary and quaternary. Explain why proteins are so sensitive to changes in temperature and pH. 8. Diagram an individual nucleotide, identify the five-carbon (pentose) sugar, the phosphate group and the nitrogenous base. Indicate with an arrow where the phosphate group of the next nucleotide would attach to build a polynucleotide. Is it a purine or a pyrimidine? Is it a DNA or an RNA nucleotide? 9. Identify examples of each of the four main classes of organic molecules and the building block components of each. 3