trends of the periodic table worksheet
advertisement

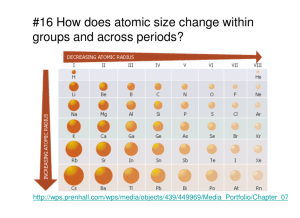
Periodic Trends Name____________________________________Per_____ ATOMIC RADIUS - Define it:_________________________________________________________ 1. What trend in atomic radius do you see as you go down a group/family on the periodic table? 2. What trend in atomic radius do you see as you go across a period/row on the periodic table? 3. Circle the atom in each pair that has the largest atomic radius. a) Al B d) Na Al b) S O e) O F c) Br Cl f) Mg Ca IONIZATION ENERGY – Define it: ____________________________________________________ 4. What trend in ionization energy do you see as you go down a group/family on the periodic table? 5. What trend in ionization energy do you see as you go across a period/row on the periodic table? 6. Circle the atom in each pair that has the greater ionization energy. a) Li Be d) Ca Ba b) Na K e) P Ar c) Cl Si f) Li K ELECTRONEGATIVITY – Define it:___________________________________________________ 7. What trend in electro-negativity do you see as you go down a group/family on the periodic table? 8. What trend in electro-negativity do you see as you go across a period/row on the periodic table? 9. Circle the atom in each pair that has the greater electro-negativity. a) Ca Ga d) Br As b) Li O e) Ba Sr c) Cl S f) O S ELECTRON AFFINITY – Define it:____________________________________________________ 10. What trend in affinity do you see as you go down a group/family on the periodic table? 11. What trend in affinity do you see as you go across a period/row on the periodic table? 12. Which of the following atoms would have the highest electron affinity? (A) Ge (B) As (C) Se (D) Sn (E) Sb 13. Which of the following atoms would have the highest electron affinity? (A) Si (B) P (C) S (D) Ge (E) As 14. Given the following: A: Ionization energy B: Electron affinity C: Atomic radius In general, as one moves across a row of the periodic table from the left to the right: (A) A, B, and C will decrease. (B) A, B, and C will increase. (C) A will increase, B and C will decrease. (D) A and B will increase, C will decrease. (E) A will decrease, B and C will increase. 22. Compare and Contrast what you know in terms of electro-negativity, electron affinity, atomic radius, ionization energy and what you discovered the trends were as you moved around the periodic table of the elements. Construct a visual aid of your choice that could help you identify the trends as you move around the periodic table of the elements.