The Periodic Table: What's the Trend?
advertisement

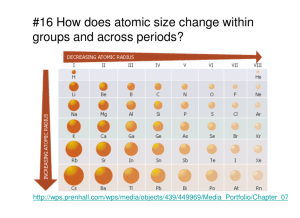
UCLA, GK-12 Science & Mathematics in Los Angeles Urban Schools http://www.nslc.ucla.edu/STEP/GK12/index.html The Periodic Table: What’s the Trend? Developed by: S. Gould, C. Skibo, H. Kang, Kim & Jennifer Adapted & Expanded from: GK-12 Lesson “Bonding: That’s what we do!!” Teacher Material California State Standards Chemistry (Atomic and Molecular Structure) 1a: Students know how to relate the position of an element in the periodic table to it’s atomic number and atomic mass 1b: Students know how to use the periodic table to identify metals, semimetals and halogens 1c: Students know how to use the periodic table to identify alkali metals, alkaline earth metals and transition metals, trends in ionization, electronegativity, and the relative sizes of ions and atoms. Synopsis The purpose of this inquiry-based lesson is to guide students to build their own periodic table based on an organization that makes sense to them. Students will then organize a traditional the periodic table and examine patterns in common data for the elements (atomic size, electronegativity, ionization energy, etc.) Background Students need to have some knowledge of subatomic particles and a concept of what an atom is before attempting this lesson. Students should have prior knowledge of the Bohr model and a brief review may be necessary before the plate models can be made. Objectives 1. Students will understand that the periodic table is highly organized 2. Students will know that elements in the periodic table are organized by increasing atomic number 3. Students will be able to identify metals and non-metals from the periodic table 4. Students will use electronic structure to understand trends in electronegativity, ionization energy, and atomic radius. Suggested Timeline Day one. Part 1: What’s the organization? 50 min. Day two. Part 2: Can you find the trend? 50 min. Materials (for each group, cost) Paper Plates Label Dots – Size appropriate (protons, neutrons and electrons) Markers (3 different colors, several of each, for ex. Black, Blue, Red) Page 1 of 7 UCLA, GK-12 Science & Mathematics in Los Angeles Urban Schools http://www.nslc.ucla.edu/STEP/GK12/index.html Butcher Paper Masking tape Pre-made element cards (name, atomic number, symbol, atomic mass, metal/non-metal, trend values). Download ElementCardsSG.doc Teacher’s Tips This lesson requires a lot of guiding questions from the instructor. Student handout will seem brief, but with the proper guidance, this lesson will give students the freedom to develop a working model of the period table. Classroom Activities – Day I 1. Warm Up Activity (5 min) a. Discuss what is organization i. Have students think of their closet or CD collections ii. Have students write several ways to organize their possessions iii. Call on several students to tell the class how they would organize their possessions b. Discuss with the class that there are several ways to organize the same stuff. It’s the same way with science there are several ways to organize data. 2. Review the Bohr model for atoms (5 min) a. NOTE: This will be the main review to help students built their plate models 3. Hand out materials, Have students build their plate models in pairs. Each pair will build one plate, see student hand out for directions to make the plate models (10-15 min) 4. Tape a piece of butcher paper to the board a. Note what period it is, each period will have a periodic table and it will be used in the second day. 5. Have the students tape their plate models to the butcher paper in no particular order (This is so that all the students can see the plate models) 6. Now have the class organize the plate models into a periodic table that make sense to them. This may require some guiding questions, some methods follow: (20-25 min) a. Method I: Guided Path i. Ask students how they would organize the elements plate models ii. Get students to brainstorm ideas for 5 minutes in their pairs iii. Report out ideas to the class iv. Let students decide what they think is the best model v. As students to go to the board and move their plate models into the organization they just decided on. b. Method II: Inquiry Path i. Tell students to organize the elements (plate models) in a way that makes sense to them. Page 2 of 7 UCLA, GK-12 Science & Mathematics in Los Angeles Urban Schools http://www.nslc.ucla.edu/STEP/GK12/index.html ii. Have students explain the reason they organized their chart the way they did as a group discussion Classroom Activities – Day II & III 1. Warm Up Activity (5-10 min) a. Review the Periodic table that was built in Day I i. What do you see? ii. Why is it organized this way? iii. Is there other ways to organize the elements? (If time permits have the students re-organize the plate models) 2. Show alternative periodic tables (see Resource Materials) 3. Review the word “Structure” 4. Ask the students “How would you organize the elements based on atomic structure?” a. Brainstorm in pairs (5 min) b. Discuss ideas with the whole class (lead students gently towards the traditional periodic table (5-10 min) c. Reorganize the elements (plate models) 5. Examine Atomic Size trends: a. Have students write the atomic size of their molecule on the butcher paper below their plate model in Black (found on element card) b. Discuss the word “Radius” c. Discuss what a “Trend” is d. Ask the students to describe the trend e. Ask student to propose ideas why the atomic radius decreases across the periodic table. f. Have students fill out their graphic organizer 6. Examine electronegativity trends: a. Have students write the electronegativity values on the butcher paper in blue b. Have students read the definition of electronegativity c. Discuss electronegativity to reinforce the definition d. Ask the students to describe the trend e. Ask the students to propose an explanation to the trend based on atomic structure (lead into octet rule) f. Have students fill out their graphic organizer 7. Examine Ionization Energy trends: a. Have students write the ionization energy values on the butcher paper in red b. Have students read the definition of ionization energy c. Discuss ionization energy to reinforce the definition d. Ask the students to describe the trend e. Ask students to propose an explanation for the trend based on atomic structure f. Have students fill out their graphic organizer 8. Let the students stick (tape) their element card to the plate model. Page 3 of 7 UCLA, GK-12 Science & Mathematics in Los Angeles Urban Schools http://www.nslc.ucla.edu/STEP/GK12/index.html a. Ask the students to look for other trends b. Talk about the trends c. Look for metals and non-metals. Are they grouped together in any way? Can you guess what an element will look like based on where it is on the periodic table? 9. Discuss the word “nobility” a. Ask for definition b. Ask how that definition could apply to elements in the periodic table. Get to a definition of “unreactive and stable” c. Look periodic table…ask students: i. Which elements are the most stable? ii. Why? iii. Explain their answer based on atomic structure Resource Materials Element Information and pictures for the “Element Cards” were used from Wikipedi.com on 9/27/05. Information was found by searching for each element individually. http://chemlab.pc.maricopa.edu/periodic/default.html is an excellent source for students to look at with several different organizations of the elements. Page 4 of 7 UCLA, GK-12 Science & Mathematics in Los Angeles Urban Schools http://www.nslc.ucla.edu/STEP/GK12/index.html (STUDENT HANDOUT BEGINS) The Periodic Table: What’s the Trend? PART I EXPLORING THE PERIODIC TABLE Introduction Today’s lab focuses on the periodic table. Using your knowledge of the atom, you will build your own periodic table. Materials Paper Plates Label dots – 3 types Markers Pre-made element card Methods In pairs, get an element card from your teacher. Using the supplies provided, build the atomic structure of your element. Using any of the information on your element card, build a periodic table with your classmates. Part I Discussion Questions 1. Describe your organization of the periodic table. 2. Why did your choose this organization? 3. Propose 2 other ways to organize the periodic table Page 5 of 7 UCLA, GK-12 Science & Mathematics in Los Angeles Urban Schools http://www.nslc.ucla.edu/STEP/GK12/index.html The Periodic Table: What’s the Trend? PART II CAN YOU SEE THE TREND? Introduction Using the periodic table you built in the last class period, you will look for patterns in the organization of the elements. Data Collection Definition Picture Trend Atomic Size Electronegativity Ionization Energy Metal Non-Metal (STUDENT HANDOUT ENDS) Page 6 of 7 UCLA, GK-12 Science & Mathematics in Los Angeles Urban Schools http://www.nslc.ucla.edu/STEP/GK12/index.html Font is Ariel Font sizes: 12 Normal for Body/Text Title – Heading 1 +Ariel 14 “Teacher Material” – Heading 2 “STUDENT HANDOUT BEGINS” – Heading 2 10 headers and footers Margins 1” on all sides Use headers and footers as shown Page 7 of 7