Chapter 7.9
advertisement

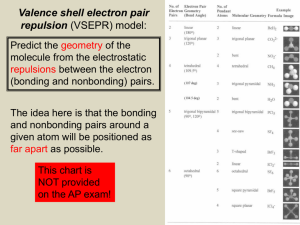
CHM 123 Chapter 7 7.9 Molecular shapes and VSEPR theory VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of atoms about a central atom in a covalent compound, or charged ion, is determined solely by the repulsions between electron pairs present in the valence shell of the central atom In the valence-shell electron-pair repulsion theory (VSEPR), the electron groups around a central atom • are arranged as far apart from each other as possible. • have the least amount of repulsion of the negatively charged electrons. • have a geometry around the central atom that determines molecular shape. Electrons in bonds and in lone pairs can be thought of as “charge clouds” that repel one another and stay as far apart as possible, this causing molecules to assume specific shapes. Working from an electron-dot structure, count the number of “charge clouds,” and then determine the molecular shape. The following are the “parent” electronic structures upon which VSEPR is based. These structures show how to minimize the energy of the structure by placing 2, 3, 4, 5 or 6 electron groups (charge clouds) as far apart around a central atom as possible in three-dimensional space. Dang 1 How many electron groups surround the central atom in the Lewis formulas? CH4 NH3 H2O In each of these examples, the electron pairs are arranged tetrahedrally, and two or more atoms are bonded in these tetrahedral directions to give the different geometries. Lone pairs are absolutely critical part of the electronic structure (charge clouds) that contributes to the shape of the molecule, but only the attached atoms are included in deriving the shape name. How many electron groups (charge clouds) are around the central atom in the following? Dang 2 Dang 3 Total # of e- groups on central atom 2 3 3 4 4 4 5 “Parent” electronic geometry # Lone pairs Idealized molecular shape Idealized bond angles Linear Trigonal Planar Trigonal Planar Tetrahedral Tetrahedral Tetrahedral Trigonal Bipyramidal # Bond ed atoms 2 3 2 4 3 2 5 0 0 1 0 1 2 0 Linear Trigonal Planar Bent Tetrahedral Trigonal Pyramidal Bent Trigonal Bipyramidal 5 Trigonal Bipyramidal 4 1 Seesaw 5 5 6 6 6 Trigonal Bipyramidal Trigonal Bipyramidal Octahedral Octahedral Octahedral 3 2 3 0 1 2 T-shaped Linear Octahedral Square Pyramidal Square Planar 180o 120 o 120 o 109.5 o 109.5 o 109.5 o 90 o, 120 o, 180 o 90 o, 120 o, 180 o 90 o, 180 o 180 o 90 o, 180 o 90 o, 180 o 90 o, 180 o 2 6 5 4 Examples: Name the shape and give the idealized bond angles for the following Lewis structure Shape Idealized bond angle Dang 4 Real bond angle Real bond angles vs. Idealized bond angles VSEPR predicts the idealized bond angle(s) by assuming that all electron groups take up the same amount of space. Since lone pairs are attracted to only one nucleus, they expand into space further than bonding pairs, which are attracted to two nuclei. As a result, real molecules that has lone pairs on the central atom often have bond angles that are slightly different than the idealized prediction. Central atom without lone pairs has the same real bond angle as the idealized angle. The exceptions to this are square planar shapes and linear (derived from trigonal bipyramidal electronic structure) shapes where the lone pairs offset one another, thus causing no deviation from ideality. Molecules with no central atom Many molecules don’t have a “central” atom but many “central” atoms. These molecules don’t fit into the shape names that we’ve learned. However, we can give an approximated shape and bond angle to each “central” atom at a time. Example: Give the approximate shape at the numbered “central” atoms Drawing 3-D structures In order to draw 3-D structure, chemists use dark wedges to indicate a bond projecting forward (out of the page) and dashed to indicate a bond going away (going back into the page) and a normal line to indicate a bond in the plane of the page Examples: Draw 3-D structure for the following molecules Dang 5