Periodicity
advertisement

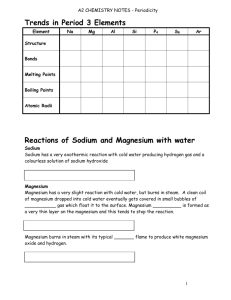
PERIODICITY REACTIONS OF PERIOD 3 ELEMENTS The Period 3 elements are: sodium, magnesium, aluminium, silicon, phosphorus, sulphur, chlorine, argon 1. WITH WATER Only sodium, magnesium and chlorine react with water under normal conditions. Sodium Sodium reacts violently with cold water. A piece of sodium dropped into water floats and immediately melts into a sphere. It fizzes, giving off hydrogen, and moves over the surface, gradually decreasing in size, until it dissolves completely. If the piece of sodium is large enough, the hydrogen will catch fire; it burns with a bright yellow flame (due to the presence of sodium vapour). The solution of sodium hydroxide remaining at the end of the reaction is colourless and alkaline. 2Na(s) + 2H2O(l) 2Na+(aq) + 2OH-(aq) + H2(g) Magnesium Magnesium reacts very slowly with cold water. Tiny bubbles of hydrogen form on the surface, and the metal slowly tarnishes. Mg(s) + H2O(l) MgO(s) + H2(g) If magnesium is heated in steam, it burns with a bright white light, releasing hydrogen and forming a white solid (magnesium oxide). Mg(s) + H2O(g) MgO(s) + H2(g) 2. WITH OXYGEN All the Period 3 elements except chlorine and argon burn in air or oxygen when ignited. The reactions are exothermic. Sodium When heated, sodium melts then burns quickly with a bright yellow flame. The product is sodium oxide, a white solid. 2Na(s) + 1/2O2(g) Na2O(s) Magnesium If magnesium is heated, it burns with a bright white light, forming white smoke and a residual white solid (magnesium oxide). 2Mg(s) + O2(g) 2MgO(s) TOPIC 13.15: PERIODICITY 1 Aluminium Aluminium in lump form is difficult to ignite, but when aluminium powder is sprinkled into a flame, it burns with a bright white light, forming white smoke and a residual white solid (aluminium oxide). 2Al(s) + 3/2O2(g) Al2O3(s) Silicon Silicon in lump form is difficult to ignite, but when grey silicon powder is sprinkled into a flame, it burns with a bright white light, forming white smoke and a residual white solid (silicon dioxide). Si(s) + O2(g) SiO2(s) Phosphorus White phosphorus is stored under water, because otherwise it could burst into flames spontaneously without heating. The usual way of igniting phosphorus is to touch it with a warm glass rod. It burns with a very bright white light, forming clouds of white smoke (phosphorus(V) oxide). P4(s) + 5O2(g) P4O10(s) Sulphur Sulphur burns slowly with a blue flame; the colour is much more pronounced when it is burnt in pure oxygen. This reaction is much less vigorous than the ones above. The product is a colourless gas with a pungent, choking odour (sulphur(IV) oxide. S(s) + O2(g) SO2(g) PROPERTIES OF THE OXIDES OF PERIOD 3 ELEMENTS Physical Properties Property m.p. /K Na2O 1548 MgO 3125 Bonding Ionic Ionic Structure Lattice Lattice Al2O3 2345 Ioniccovalent Lattice SiO2 1883 P4O10 573 SO2 200 Covalent Covalent Covalent Macromolecular Molecular Molecular The ionic lattices, Na2O and MgO, have strong interionic forces in three dimensions and have high melting points. Silicon dioxide, which is macromolecular, has strong covalent bonding in three dimensions and has a high melting point. Aluminium oxide, which has an intermediate ionic-covalent lattice, has strong bonds in three dimensions and has a high melting point. The two molecular substances, P4O10 and SO2, have only weak intermolecular forces (dipole-dipole) and therefore have low melting points. TOPIC 13.15: PERIODICITY 2 Chemical Properties Sodium oxide When added to water, sodium oxide dissolves to form an aqueous solution containing sodium hydroxide. The solution is, therefore, alkaline and has a pH of 14. Na2O(s) + H2O(l) 2Na+(aq) + 2OH-(aq) Magnesium oxide Magnesium oxide is sparingly soluble in water, but when it is added to water, enough dissolves to form a weakly alkaline solution. The pH is about 9. MgO(s) + H2O(l) Mg2+(aq) + 2OH-(aq) Aluminium oxide Aluminium oxide is insoluble in water and does not react with water. When it is added to water, the resultant pH is 7. Silicon dioxide Silicon dioxide is insoluble in water and does not react with water. When it is added to water, the resultant pH is 7. Phosphorus(V) oxide Phosphorus(V) oxide is very soluble in water and reacts violently with it. The product is phosphoric(V) acid, H3PO4, a strong acid. The pH of the resultant solution is about 0. P4O10(s) + 6H2O(l) 4H3PO4(aq) Sulphur(IV) oxide Sulphur(IV) oxide is fairly soluble in water and dissolves to form sulphuric(IV) acid (also called sulphurous acid), H2SO3, a weak acid. The pH of the solution is about 3. SO2(g) + H2O(l) H2SO3(aq) The properties of the oxides change across the period. The ionic metal oxides on the left hand side are basic. The non-metal oxides on the right hand side are acidic. Aluminium oxide, which is in the middle, is amphoteric. Acidic oxides can react with bases to produce a salt and water. SiO2 + 2NaOH P4O10 + 12NaOH Na2SiO3 + H2O 4Na3PO4 + 6H2O Basic oxides can react with acids to produce salt and water also. MgO + H2SO4 TOPIC 13.15: PERIODICITY 3 Mg SO4 + H2O