this Outline - Alcohol Medical Scholars Program
advertisement

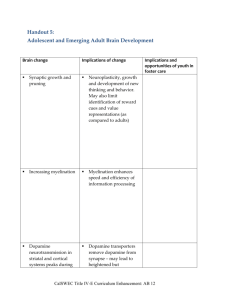
Impaired Decision Making in Substance Use Disorders Claire E. Wilcox, MD Department of Psychiatry University of New Mexico Alcohol Medical Scholars Program (Slide 1) I. Introduction A. Case Presentation (Slide 2) 1. Male physician, age 55 2. Intelligence is high a. Was employed at a university in past b. Ivy league-trained c. Now unemployed 3. Methamphetamine dependent a. Residential treatment 3X b. Intensive outpatient 6 months 1X c. Repeated relapses d. Wants to quit methamphetamine B. Has made poor/maladaptive decisions despite (Slide 3) 1. High IQ 2. General self-control 1 C. Was a result of poor decision-making which contributed to 1. Initial use of substances 2. Escalation of problems 3. Repeated relapses: (this will be the focus of this talk) E. This lecture covers (Slide 4) 1. Neurocog (thought processes) and decision-making 2. Neuroanatomical and neurochemical systems involved 3. How systems can malfunction in substance use disorder (SUD) 4. Treatment implications II. Neurocognitive perspective on decision making: (e.g., carrots vs cookies) A. When choosing between carrots & cookies (or drugs vs abstinence) 1. How is decision made? (Slide 5) a. Process occurs inside the brain b. Involves 3 stages c. Each stage affects the next d. Experience driven 2. The 3 stages are1: a. Stage 1: Assessment (Slide 6) (Same picture will recur later when Stage 1 discussed) 1’. See/smell items a’. Cookie with melted chips b’. Carrot c’. Item = ‘cue’ or ‘stimulus’ 2 2’. Form a preference a’. Stimulus (eg. cookie) wanting 1’. Wanting has direction (or valence) a’’. Positive b’’. Negative 2’. Wanting has intensity (or salience) a’’. Low salience for carrot b’’. High salience for cookie b’. Context (environment) wanting 1’. If had good cookies here before 2’. Then wanting b. Stage 2: Action (Slide 7) (Same picture will recur later when Stage 2 discussed) 1’. Action selection (take a bite of cookie or use drug) 2’. Perform action (bring cookie to mouth) 3’. Complete action (chew/swallow) c. Stage 3: Experience outcome (Slide 8,9) (Stage 3 = Effect PLUS Learning) (Same two pictures will recur later when Stage 3 discussed) 1’. Evaluation/feedback (yummy!!!) a’. Person has an experience of eating/using b’. Can be positive, negative, neutral c’. Outcome of eating influences learning 3 2’. Learning (involving conditioning) a’. Influences future decisions b’. Classical conditioning 1’’. Pavlov’s dog: bell with food 2’’. Begin salivate with bell (cue) alone c’. Operant conditioning 1’’. Skinner’s pigeons 2’’. Punishment decreases behavior 3’’. Reward increases behavior 4’’. Relief of negative increases behavior 5’’. No consequence yields extinction B. What influences/modifies each stage? 1. Influence on Stage 1: assessment 1 (Slide 10) a. Stimulus (cue) = sight, smell of cookie/carrot (or drug) b. Environment (context) good prior effect of cookies/drugs c. Emotional state 1’. Hunger/wanting 2’. Stress & past relief by food/drugs 3’. Mood 4’. Drug state & related impaired judgment a’. Intoxication b’. Withdrawal d. Preexisting psychological processes cookie/drug 4 1’. Impulsivity 2-4 a’ Ability to postpone reward b’ Risk taking/sensation seeking c’. Tendency to act rashly/urgency 2.’ Emotional regulation processes 3’. Stimulus driven attention 5,6 a’. Ability to attend to environmental stimuli b’. This is a neurocognitive process 4’. Craving processes 5’. Processes involved in cost-benefit analysis 2. Stage 2: What affects action1? (Slide 11) a. Preference from Stage I (despite diet, I prefer cookie/drug) b. Moderating psychological processes / traits 1. Degree of flexibility a’. Flexible consider options b’. That not stuck on wanting cookie/drug d’. That relapse 2. Degree of inhibitory/cognitive control relates to5,6 a’. Selective attention 1’. Conflict 2’ That focus on key aspect of task b’. Sustained attention ability to inhibit act 1’. Eg. read a newspaper 5 2’. Not get distracted by noise 3. Stage 3: Influences on experience of outcome1 (Slide 12) a. Action from Stage 2 b. Experience of outcome (what is it like to perform action) 1’. Euphoria (soooo tasty/rewarding) 2’. Disappointment/regret/guilt (neg. consequences) b. Time between taking item and reward learning c. Moderating psychological processes / traits: 1’. Processes encoding reward (how good it felt) 2’. Ability to process pros & cons of the choice made (eg. to procure cookie/drug) a’. How the brain evaluates future action b’. Involves error calculation 1’’. Actual vs. expected value 2’’. Was outcome similar to expectation? c’. Learning updates stimulus (eg. cookie/drug) value C. Similar 3 stages of decision making seen in the patient (Slide 13) 1. Assessment a. Saw someone smoking methamphetamine (stimulus) b. Formed a preference c. His experiences/emotional state/traits strong preference 1’. Past learning that methamphetamine = rewarding 6 2’. Stressed because recently fired again 3’. Context: party where he’d used before 4’. Higher impulsivity at baseline 2. Action a. Poor baseline sustained attention b. Made a decision to take drug c. Took drug 3. Learned from experience a. Drug feels good b. Feelings of stress c. Likelihood of preference for drug in future d. Strength of stimulus to cause action to take drug F. These stages reflected changes in CNS function; that’s next topic G. This has lecture covered (Slide 14) 1. Neurocog aspects of decision making 2. Now we move on to: a. Neurochemical systems in decision-making b. Neuroanatomical systems in decision-making IV. Neuroanatomy and neurochemistry of decision-making A. All traits and decision processes have chemical components B. Effects of chemistry differ in different brain regions C. Neurochemical systems/neurotransmitters and decision-making 1. Dopamine (DA): Key effects on 7-10 (Slide 15) 7 a. Salience (intensity of wanting) (Stage 1) b. Cost benefit analysis (Stage 1) c. Attention 1’. Stimulus-driven (Stage 1) 2’. Selective (Stage 2) 3’. Sustained (Stage 2) d. Action initiation and selection (Stage 2) e. Associative learning/conditioning (Stage 3) 1’. Encodes ‘high’ 2’. Reinforces a behavior (learning) 2. Noradrenaline (NA) key effects on9,11-14 (Slide 16) a. Stress response (Stage 1) 1’. Sympathetic nervous system a’. In CNS: ‘Fight or flight’ b’. Outside of brain: pulse, sweating, etc 2’. Brain stress response CRF b. Optimizing performance and focus (Stage 2) c. Facilitating exploration of options (Stage 2) d. Learning about decision outcomes (Stage 3) 3. Glutamate (Glu) key effects on (Slide 17) a. Cue-elicited behavioral activation 1’. Preference formation (Stage 1) 2’. Action selection (Stage 2) 8 b. Learning (Stage 3) D. A review of neuroanatomy of decision making (Slide 18) 1,15,16 1. Dorsal Striatum (DS) a. Location: subcortical b. Functional subregions = caudate and putamen c. Major connections related to decision making are 1’. Substantia nigra (SN) 2’. Prefrontal cortex (PFC) & anterior cingulate (ACC) d. Works in relation to decision making by 1’. Encode cue value (Stage 1) 2’. Action selection and execution (Stage 2) a’. Parkinson’s disease movement probs b’. = A disease of dorsal striatum 2. Ventral Striatum (VS) a. Location: subcortical b. Functional subregions = nucleus accumbens c. Major connections related to decision making are 1’. Ventral tegmental area (VTA) 2’. PFC and ACC 3’. Amygdala d. Works in relation to decision making by 1’. Encode cue value (Stage 1) 2’. Encode reward value (Stage 3) 9 3. Prefrontal cortex (PFC) and anterior cingulate cortex (ACC) a. Location 1’. PFC is front part of brain a’. Dorsolateral (DLPFC) b’. Orbitofrontal (OFC) 2’. ACC goes along corpus callosum b. Major connections related to decision making are 1’. Ventral tegmental area 2’. Striatum (dorsal and ventral) 3’. Amygdala c. Works in relation to decision making by 1’. Encode cue value (Stage 1) 2’. ‘Higher order’ preference assignment (Stage 1) 3’. Attention (Stage 1 & 2) 4’. Inhibitory control (Stage 2) 5’. Encode drug or reward value (Stage 3) 4. Example of actual data from fMRI studies17,18 (Slide 19) 5. Neuroanatomy by neurotransmitter a. CNS regions rich in DA include (Slide 20) 1’. Cell bodies (where it comes from) a’. Ventral tegmental area (VTA) b’. Substantia nigra (SN) 2’. Where it’s released 10 a’. Striatum (dorsal and ventral) b’. Prefrontal cortex (PFC) c’. Anterior cingulate cortex (ACC) 3’. Plays important role in a’. Stimulus processing/preference (Stage 1) b’. Action selection and execution (Stage 2) c’. Reward encoding (Stage 3) d’. Learning (Stage 3) b. CNS regions rich in NA (Slide 21) 1’. Locus coeruleus (cell bodies) 2’. Where it’s released a’. Amygdala b’. Prefrontal cortex (PFC) c’. Anterior cingulate cortex (ACC) 3’. Plays important role in a’. Stress response (Stage 1) b’. Action selection and execution (Stage 2) c’. Learning (Stage 3) c. CNS regions rich in Glu (Slide 22) 1’. Prefrontal cortex (PFC) (cell bodies) 2’. Released in a’. Striatum b’. Amygdala 11 2’. Plays an important role in a’. Stimulus processing/preference (Stage 1) b’. Action selection (Stage 2) c’. Learning (Stage 3) 6. Probs in these systems impaired decision-making in SUD F. This lecture has covered (Slide 23) 1. Neurocog aspects of decision-making 2. Neuroanatomy and neurochemical systems involved 3. Now we go on to how systems malfunction in SUD V. Maladaptive decision-making in SUD A. SUD have maladaptive decision-making processes (Slide 24) 1. Results from 1+ stages functioning poorly 2. Problems reflect biological changes 3. Those maladaptive decision-making a. Maladaptive = decisions not maximizing future success b. Relapse risk 4. Can reflect maladaptive at baseline (eg. geneticsimpulsive)19,20 5. Also reflect prior drug use a. Eg. of role of dopamine7 1’. Chronic drugs changes in DA function including: a’. DA release to reward (not to stimulus) b’. DA receptors (DRD2) c’. Direct toxicity to DA neurons (stimulants) 12 2’. DA changes maladaptive decision making b. Eg. of role of noradrenaline (NA) and CRF 13 1’. Chronic drugs and repeated withdrawal a’. NA release to stress b’. CRF release to stress c’. Negative emotion to stress 2’. NA/CRF changes maladaptive decision making B. Decision making problems by stage (e.g., doc using meth) 1,14-16,21 1. His Stage 1 (assessment) in relapse: wants drug now (Slide 25) a. Drug cue valence (direction) and salience (intensity) 1’. Drug cue has positive value to him 2’. Drug cue has more intense effect 3’. CNS activation to drug cue in drug-users5,14-16,21 4’. DA and Glu release to drug cue in drug-users 5’. Those reflect past conditioning from drug use 6’. Explains relapse b. Prefers short-term reward (the high) reflecting impulsivity 22 1’. Prior impulsivity also contributes to relapse 2’. Value of fast reward 3’. Difficult for him to delay reward (waiting) 4’. Contributes to relapse 13 c. Drug cue salience during stress 1’. Drug feeling of stress and neg. emotion 2’. Reflects Skinner’s operant conditioning a’. Things that bad feelings are rewarding b’. Has learned that drug neg. emotion c’. Negative emotions drug value 3’. Process reflects NA and stress hormones23 a’. CRF and NA release to stress b’. Negative emotions to stress c’. That craving 4’. Contributes to relapse 2. Stage 2: For above case is hard to inhibit acting on “want” (Slide 26) a. His usual/habitual actions are favored7,24 1’. Less flexibility/can’t see other options 2’. Reflects drug cues DA in dorsal striatum 4’. Contributes to relapse b. He also has inhibitory control of action 1. Acts despite potential negative consequences 2. Brain activation during inhibitory control 25,26 3. DA receptors (DRD2) in striatum may contribute7 4. When drug available, may ‘forget’ to stay clean14 5. Contributes to relapse 14 3. Stage 3: case’s imbalanced reward encoding/learning (Slide 27) 7,27 a. When first used: felt drug reward 1. Led to conditioned learning 2. Caused effect of seeing drug cue in future 3. DA & Glu in ventral striatum important in learning b. While dependent on drug: drug reward/dose7 1’. Less dopamine release/dose over time 2’. That reward to drug/dose over time 3’. Experienced value < expected value 4’. To further drug use b. Learning from negative consequences 1’. Fail to consider SUD associated problems 2’. Striatal DA receptor (DRD2) may be a cause20 3’. Contributes to relapse C. Tieing it all together: example of the case by stage for relapse (Slide 28) 1. Stage 1: Assessment A. Preference for drug when sees stimulus B. Reflects DA and Glu release in striatum C. Reflects brain reactivity to seeing drug cue D. If stressed: brain CRF, NA release 1’. That neg. emotion 15 2’. This desire for drug to remove neg. emotion 3’. That want drug E. Related to impulsivity (genetics) 2. Stage 2: Action A. Chooses to use drug, and uses it B. Reflects flexibility/ DA in dorsal striatum C. That reflects inhibitory control/ fcn ACC & PFC 3. Stage 3: Outcome evaluation A. Reward processing7 1’. Drug is rewarding: it d stress & improved mood 2’. But was, not as rewarding as expected 3’. This due to DA release in ventral striatum 4’. Leads to further drug use to get the “old feeling” B. Learning 1’. Hasn’t learned from negative consequences like: a’. Criticized at work b’. Financial problems 2’. Could reflect DA receptor (DRD2) in striatum D. This lecture has covered (Slide 29) 1. Background 2. Neurocog aspects of decision making 3. Neuroanatomical and neurochemical systems 4. How systems malfunction in SUD 16 5. Treatment implications VI. Treatment implications of impaired decision-making A. Prevention through (Slide 30): 1. Limit/prevent exposure to drugs a. Protect brain circuitry that contribute to use b. Prevent lasting CNS changes that perpetuate use 2. Target high-risk people including a. Impulsive adolescents (have impulse control) b. Those with genetic predisposition re SUD or impulsivity c. Those with cognitive problems 1’. Traumatic brain injury 2’. Schizophrenia d. Those with problems in stress response system 1’. Post traumatic stress disorder 2’. Depressive syndromes 3’ Major anxiety syndromes B. Treatment of SUD through (Slide 31): 1. Adaptive decision-makingrelapse 2. Methods to improve decision-making a. Medications 1’. Block brain response to stimulus 2’. Improve cognitive control 3’. Decrease drug reward/learning 17 b. Psychotherapy 1’. Increases adaptive decision-making 2’. Examples a’. Contingency management therapy b’. Relapse prevention/cognitive behavior c’. Other (motivational interviewing, cog rehab) d’. Details given in other lectures C. Examples of current/future SUD medications and how they work 1. Stage 1: a. Strength of stimulus (e.g., naltrexone [ReVia]) (Slide 32) 1’. FDA approved for alcohol use disorders (AUDs) 2’. Brain response to alcohol pictures 28 3’. Alcohol craving 4’. Alcohol drinking 5.’ Blocks DA by blocking opioid 6’. (Could argue that it also works at stage 3) b. Negative emotional states, including (Slide 33): 1’. Withdrawal (e.g., methadone [Methadose]) a’. FDA approved for opiate use disorders b’. Withdrawal anxiety/craving all day c’. ”Normalizes” stress response 23,29 1’. Corticotrophin releasing factor 18 a’’. Brain hormone b’’. In amygdala in stress c’’. CRF stimulates cortisol c’’. Cortisol=main stress hormone 2’. Norepinephrine release to stress 2’. Mood/anxiety major disorders a’. When treat psychiatric disorders b’. Drug craving to relieve bad feelings 2. Stage 2: cog function inhibitory control (Slide 34) a. Meds may impact- eg. of varenicline (Chantix) for cigs30 b. Cog enhancers under study for SUDs31 1. Eg. of memantine 2. An approved Rx for Alzheimers Dz 3. Stage 3: drug reward (Slide 35) a. Vaccines 1’. Under development a’. Cocaine/nicotine 1”. Create antibody to coke/nicotine 2” Antibody/drug can’t cross to brain 3’’. Blocks reward/learning D. Examples of psychotherapies (Slide 36) 1. Contingency management therapy 32 a. Based on operant conditioning theory 19 b. Pay patient for negative urines c. Pay for good behavior changes decisions 1’. Choose abstinence b/c rewarded 2’. Learn abstinence brings reward 3’. More likely to stay abstinent over time 2. Relapse prevention therapies/ cognitive behavior therapy 33 a. Individual identifies relapse triggers b. Example of case’s relapse triggers: 1’. Cues (person smoking methamphetamine) 2’. Context (party) 3’. Negative emotions (stress) c. Learns to avoid triggers to decrease relapse 1’. Recall cues trigger cascade of events drug use 2’. By avoiding triggers don’t trigger cascade d. This decreases risk of relapse VII. Conclusion A. This lecture covered (Slide 37) 1. Background 2. Neurocog aspects of decision making 3. Neuroanatomical and neurochemical systems involved 4. How systems can malfunction in SUD 5. Treatment implications 20 References 1. Ernst M, Paulus MP (2005): Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol Psychiatry. 58:597-604. 2. Winstanley CA, Dalley JW, Theobald DE, Robbins TW (2004): Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 29:1331-1343. 3. Dawe S, Gullo MJ, Loxton NJ (2004): Reward drive and rash impulsiveness as dimensions of impulsivity: implications for substance misuse. Addict Behav. 29:13891405. 4. Dawe S, Loxton NJ (2004): The role of impulsivity in the development of substance use and eating disorders. Neurosci Biobehav Rev. 28:343-351. 5. Nestor L, McCabe E, Jones J, Clancy L, Garavan H (2011): Differences in "bottom-up" and "top-down" neural activity in current and former cigarette smokers: Evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. Neuroimage. 56:2258-2275. 6. Medendorp WP, Buchholz VN, Van Der Werf J, Leone FT (2011): Parietofrontal circuits in goal-oriented behaviour. Eur J Neurosci. 33:2017-2027. 7. Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F (2011): Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 108:15037-15042. 8. Assadi SM, Yucel M, Pantelis C (2009): Dopamine modulates neural networks involved in effort-based decision-making. Neurosci Biobehav Rev. 33:383-393. 9. Rogers RD (2011): The roles of dopamine and serotonin in decision making: evidence from pharmacological experiments in humans. Neuropsychopharmacology. 36:114-132. 10. Fields HL, Hjelmstad GO, Margolis EB, Nicola SM (2007): Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 30:289-316. 11. Aston-Jones G, Cohen JD (2005): An integrative theory of locus coeruleusnorepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 28:403-450. 12. Yu AJ, Dayan P (2005): Uncertainty, neuromodulation, and attention. Neuron. 46:681-692. 13. Koob GF (2009): Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry. 42 Suppl 1:S32-41. 14. Kalivas PW, Volkow ND (2005): The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 162:1403-1413. 15. Liu X, Hairston J, Schrier M, Fan J (2011): Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 35:1219-1236. 16. Diekhof EK, Falkai P, Gruber O (2008): Functional neuroimaging of reward processing and decision-making: a review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res Rev. 59:164-184. 17. Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR (2011): Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend (see appendix). 21 18. Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE (2011): Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 36:2086-2096. 19. Mitchell SH (2011): The genetic basis of delay discounting and its genetic relationship to alcohol dependence. Behav Processes. 87:10-17. 20. Klein TA, Neumann J, Reuter M, Hennig J, von Cramon DY, Ullsperger M (2007): Genetically determined differences in learning from errors. Science. 318:16421645. 21. Wilson SJ, Sayette MA, Fiez JA (2004): Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 7:211-214. 22. Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, et al. (2008): Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology (Berl). 201:183-193. 23. Koob GF (2009): Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 56 Suppl 1:18-31. 24. Everitt BJ, Robbins TW (2005): Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 8:1481-1489. 25. Kaufman JN, Ross TJ, Stein EA, Garavan H (2003): Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 23:7839-7843. 26. Garavan H, Hester R (2007): The role of cognitive control in cocaine dependence. Neuropsychol Rev. 17:337-345. 27. Mallett KA, Lee CM, Neighbors C, Larimer ME, Turrisi R (2006): Do we learn from our mistakes? An examination of the impact of negative alcohol-related consequences on college students' drinking patterns and perceptions. J Stud Alcohol. 67:269-276. 28. Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K (2008): Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 65:466-475. 29. Stimmel B, Kreek MJ (2000): Neurobiology of addictive behaviors and its relationship to methadone maintenance. Mt Sinai J Med. 67:375-380. 30. Shim JC, Jung DU, Jung SS, Seo YS, Cho DM, Lee JH, et al. (2011): Adjunctive Varenicline Treatment with Antipsychotic Medications for Cognitive Impairments in People with Schizophrenia: A Randomized Double-Blind Placebo-Controlled Trial. Neuropsychopharmacology. 31. Brady KT, Gray KM, Tolliver BK (2011): Cognitive enhancers in the treatment of substance use disorders: clinical evidence. Pharmacol Biochem Behav. 99:285-294. 32. Weinstock J, Alessi SM, Petry NM (2007): Regardless of psychiatric severity the addition of contingency management to standard treatment improves retention and drug use outcomes. Drug Alcohol Depend. 87:288-296. 33. Kadden R, Cooney NL (2007): Treating alcohol problems. In: Marlatt, G.A., Donovan, D.M. (Eds.), Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. Guilford Press, New York, pp. 65–91. 22