Haber-Bosch Process: Le Châtelier's Principle & Ammonia
advertisement

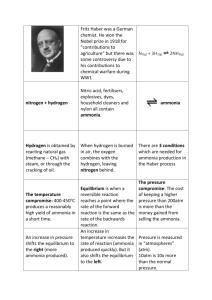
The Haber-Bosch Process An Application of the Le Châtelier’s Principle Virtually all of the world’s ammonia (NH3) is produced via the Haber-Bosch process and almost all of our inorganic nitrogen compounds (nitrates, nitrites etc) are synthesized from this ammonia production. About 10 million tons of nitric acid is produced annually from ammonia. Nitric acid is used to produce ammonium nitrate fertilizers, nitro explosives, plastics and dyes. In 1910, German chemist, Fritz Haber developed the equipment and procedures for producing ammonia from its corresponding elements; N2 and H2. He later received a Nobel Prize in chemistry for his work. Later, Carl Bosch further developed the process to convert it into industrial scale. exo N2 (g) + 3H2 (g) 2NH3 (g) ∆H = -92.4 kJ 4mog endo 2 mog Consider a scenario where a chemist wishes to make NH3 in the lab by allowing a single set of reactants to reach equilibrium in a closed container. An optimal temperature and pressure would be ideal so that both percent yield and reaction rate need to be considered. (i) To produce higher percent yield of NH3 at equilibrium, what kind of temperatures would be needed? Prediction: cold temperature Haber-Bosch Process: 600°C (ii) How would this affect the forward and reverse reaction rates? Prediction: cold temp: lower rxn rates, fewer particles with sufficient energy, low yield of ammonia Haber-Bosch Process: High temperature enables both forward and reverse rates to take place creates more effective collisions (iii) To produce higher percent yields of NH3, what kind of pressure would be needed? Prediction: high pressure Haber-Bosch Process: 200 atm (iv) What is “fixing” nitrogen? Bonding nitrogen gas with other substances to make compounds. In nature: .lightening from thunderstorms produces NO2 .pea plants produces NO2 (v) What catalysts were used in the Haber Process? uranium and osmium (vi) What is the yield of ammonia production? 8% (vii) Name 3 stresses in this process that affect Le Châtelier’s Principle and that contribute in the production of ammonia. 1) Temperature: high to have particles going fast enough 2) Pressure: high, shift to product side (4 mog into 2 mog) 3) Remove ammonia as fast as it is formed, forcing system to produce more (vii) Why was Fritz Haber’s Nobel Prize contested? Prolong WWI by 2-3 years Haber created mustard gas .responsible for killing thousands of people (viii) What became of Fritz Haber? Homeless in fear of going to concentration camp for being Jewish