Ammonia - WordPress.com
advertisement
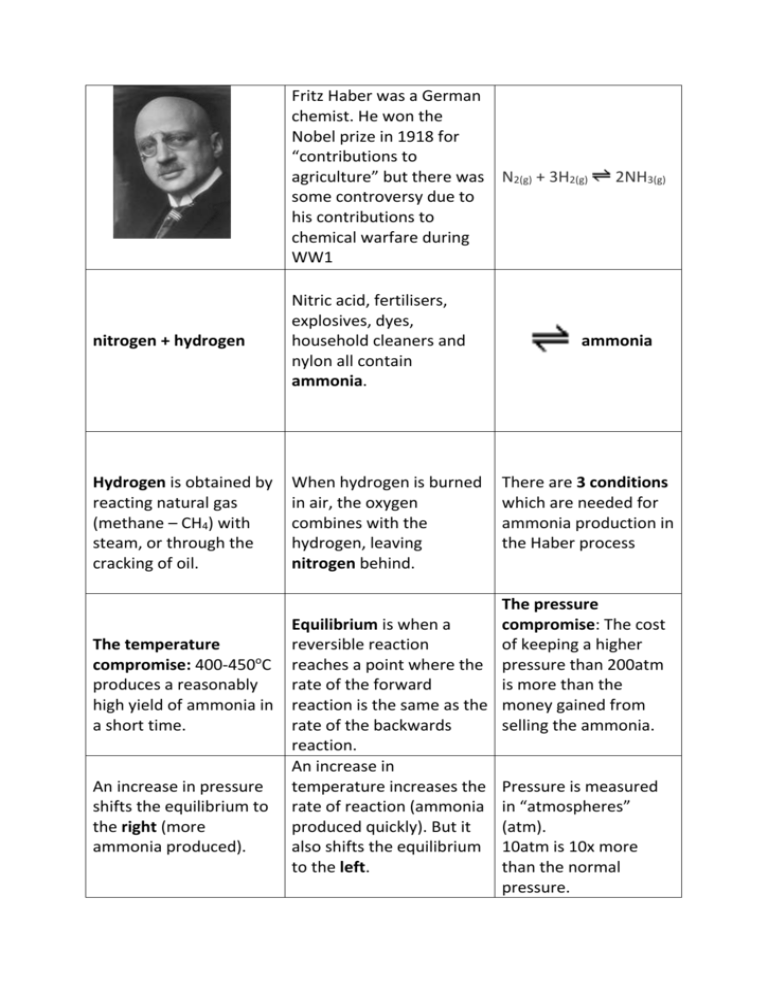
Fritz Haber was a German chemist. He won the Nobel prize in 1918 for “contributions to agriculture” but there was N2(g) + 3H2(g) some controversy due to his contributions to chemical warfare during WW1 nitrogen + hydrogen Nitric acid, fertilisers, explosives, dyes, household cleaners and nylon all contain ammonia. Hydrogen is obtained by reacting natural gas (methane – CH4) with steam, or through the cracking of oil. When hydrogen is burned in air, the oxygen combines with the hydrogen, leaving nitrogen behind. 2NH3(g) ammonia There are 3 conditions which are needed for ammonia production in the Haber process The pressure Equilibrium is when a compromise: The cost The temperature reversible reaction of keeping a higher o compromise: 400-450 C reaches a point where the pressure than 200atm produces a reasonably rate of the forward is more than the high yield of ammonia in reaction is the same as the money gained from a short time. rate of the backwards selling the ammonia. reaction. An increase in An increase in pressure temperature increases the Pressure is measured shifts the equilibrium to rate of reaction (ammonia in “atmospheres” the right (more produced quickly). But it (atm). ammonia produced). also shifts the equilibrium 10atm is 10x more to the left. than the normal pressure. A catalyst of iron is used. It doesn’t increase the yield of ammonia produced, only the rate of reaction. In the Haber Process a compromise has to be made between pressure and temperature to gain the greatest, most economical yield. Which is better – more ammonia (high yield) produced very slowly, or a small amount (low yield) produced quickly? Ammonia is collected as a gas. At this point the temperature is lowered and it turns into a liquid. When the gaseous ammonia is turned into a liquid, the unused hydrogen and nitrogen stay as a gas and are recycled. Taking the liquid ammonia away stops the reaction going backwards. Who invented the Haber process? What does it produce? Why do we need it? How does it work? When does it produce the highest, most economical yield?