The Haber Process
advertisement
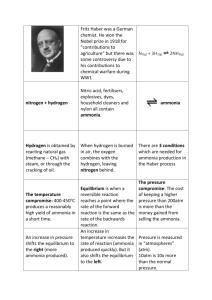
The Haber Process An essential industrial process The Haber Process This reaction makes ammonia out of hydrogen and nitrogen. The nitrogen comes from the air (78% N). You don’t need to worry about where the hydrogen comes from! The Haber Process The Haber process is a REVERSIBLE reaction N2(g) + 3H2(g) nitrogen + hydrogen 2NH3(g) (+ heat) ammonia A reversible reaction is one where the products of the reaction can themselves react to produce the original reactants. The Haber Process The Haber Process You need to LEARN the industrial conditions this reaction occurs in off by heart – this is a favourite exam question!!! Industrial conditions: PRESSURE: TEMPERATURE: CATALYST: 200 atmospheres 4500C Iron The Haber Key facts H and N are mixed in a 3:1 ratio 2. Because the reaction is reversable not all the nitrogen and hydrogen will convert to ammonia. 3. The ammonia forms as a gas but cools and liquefies in the condenser 4. The H and N which do not react are passed through the system again so they are not wasted. 1.




![mic_class10_10.ppt [Compatibility Mode]](http://s3.studylib.net/store/data/008220705_1-eb9498ce6cd0ab3209762ef99981a3a3-300x300.png)