Haber process

Making Fertilisers
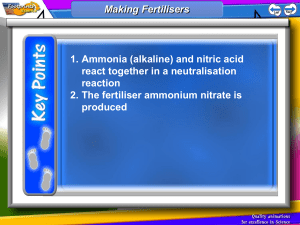
1. Ammonia (alkaline) and nitric acid react together in a neutralisation reaction
2. The fertiliser ammonium nitrate is produced
Making fertilisers
Making Fertilisers
Making fertilisers
Neutralisation
Making fertilisers
The Haber Process
1. Ammonia is made in the Haber process
2. The raw materials are nitrogen (from the air) and hydrogen (from natural gas)
3. The conditions needed are;
• high temperatures (about 450 0 )
• high pressures (200 atm).
• iron catalyst
The Haber Process
The Haber Process
The Haber Process
The Haber Process
The Haber Process
Reversible reactions
1. The Haber process reaction is reversible
2. This means that the product (ammonia) can break down again into the reactants
(nitrogen and hydrogen)
Reversible reactions
Reversible reaction
Reversible reaction
Equilibrium
1. For a reversible reaction in a closed system, an equilibrium is reached where the rate of the forward reaction equals the rate of the reverse reaction
2. The amount of reactants and products in the equilibrium depends on the conditions
3. The equilibrium will move to reduce any changes in the reaction
Equilibrium
Equilibrium
Equilibrium
Low temperature
Low temperature
High temperature
Hign temperature
Making Nitric acid
1. Ammonia reacts with oxygen in the air in the presence of a hot platinum catalyst to produce nitrogen monoxide
2. The nitrogen monoxide reacts with water and more oxygen to produce nitric acid
Making nitric acid
Making Nitric Acid
Making nitric acid
Quiz
Quiz