Ionic Bonding Puzzle Lab: Chemical Formulas & Compounds
advertisement

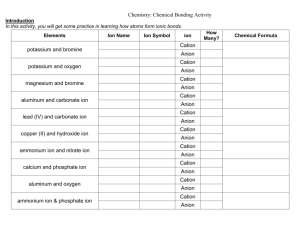
Ionic Bonding Puzzle Lab Introduction When metals and non-metals chemically react, the atoms will tend to form ions or charged atoms. Ions form because electrons are either gained or lost. Metals will generally form cations or positive ions, since they tend to donate electrons Non-metals will form anions or negative ions, since they tend to accept electrons. Activity In this activity you will create models of ionic compounds and observe the chemical formula of the binary molecules you have created. Your data will be recorded in a data table or chart, similar to the one below for each compound you create. Procedures: 1. Cut the puzzle pieces. 2. Organize them in two groups: anions (negative ions) and cations (positive ions). 3. Use the name on the puzzle pieces to pick the appropriate parts for the first compound in the chemical compound table. 4. Glue the puzzle pieces that you picked on the result sheet to form the compound. 5. Determine the name of each compound you created. 6. Draw and fill out a Data Chart under the compound. 7. Repeat steps 3-6 to construct the compounds in the chemical compounds page. Chemical Compounds Table 1. MgF2 2. LiCl 3. NaCl 4. K2S 5. Cs2O 6. Li3P 7. FeBr3 8. CaCl2 9. Ag2S 10. SnF4 11. Na3N 12. CuI3 13. K3N 14. MgO 15. CoBr3 16. CaO 17. FeCl3 18. NiS 19. NaI 20. BeO Data Chart # of Electron Lost/gained Metal------------Non-Metal------ Ion (Anion/Cation) Chemical Formula Name # of Electron Lost/gained Ion Chemical Formula Name Chemical Formula Name Chemical Formula Name Chemical Formula Name Chemical Formula Name Chemical Formula Name (Anion/Cation) Metal------------Non-Metal-----# of Electron Lost/gained Ion (Anion/Cation) Metal------------Non-Metal-----# of Electron Lost/gained Ion (Anion/Cation) Metal------------Non-Metal-----# of Electron Lost/gained Ion (Anion/Cation) Metal------------Non-Metal-----# of Electron Lost/gained Ion (Anion/Cation) Metal------------Non-Metal-----# of Electron Lost/gained Metal------------Non-Metal------ Ion (Anion/Cation) # of Electron Lost/gained Ion Chemical Formula Name Chemical Formula Name Chemical Formula Name Chemical Formula Name Chemical Formula Name Chemical Formula Name Chemical Formula Name (Anion/Cation) Metal------------Non-Metal-----# of Electron Lost/gained Ion (Anion/Cation) Metal------------Non-Metal-----# of Electron Lost/gained Ion (Anion/Cation) Metal------------Non-Metal-----# of Electron Lost/gained Ion (Anion/Cation) Metal------------Non-Metal-----# of Electron Lost/gained Ion (Anion/Cation) Metal------------Non-Metal-----# of Electron Lost/gained Ion (Anion/Cation) Metal------------Non-Metal-----# of Electron Lost/gained Metal------------Non-Metal------ Ion (Anion/Cation) # of Electron Lost/gained Ion Chemical Formula Name Chemical Formula Name Chemical Formula Name Chemical Formula Name Chemical Formula Name Chemical Formula Name (Anion/Cation) Metal------------Non-Metal-----# of Electron Lost/gained Ion (Anion/Cation) Metal------------Non-Metal-----# of Electron Lost/gained Ion (Anion/Cation) Metal------------Non-Metal-----# of Electron Lost/gained Ion (Anion/Cation) Metal------------Non-Metal-----# of Electron Lost/gained Ion (Anion/Cation) Metal------------Non-Metal-----# of Electron Lost/gained Metal------------Non-Metal------ Ion (Anion/Cation) Analysis and Conclusion Questions 1.) What is a chemical bond? 2.) How does a chemical bond occur? 3.) What do atoms gain by bonding? 4.) Contrast cations and anions. 5.) When is it appropriate to use Roman numerals in naming compounds? 6.) What does the numerical subscript (such as N2) following an element in a chemical formula mean? 7.) What does a numerical subscript following a set of parenthesis (SO4)2 in a chemical formula mean? 8.) In general, ionic bonds occur when what two classes of elements combine? 9.) What is the overall charge an ionic compound contains? F-1 Fluoride Y+3 Yttrium(III) F-1 Fluoride K+1 Potassium Cl-1 Chloride K+1 Potassium Cl-1 Chloride Fe+3 Iron(III) Sc+1 Cesium(I) K+1 Potassium Cl-1 Chloride Cl-1 Chloride Cl-1 Chloride Br-1 Bromide Br-1 Bromide Br-1 Bromide Ca+2 Calcium Br-1 Bromide Mg+2 Magnesium Br-1 Bromide Mg+2 Magnesium Ag+1 Silver(I) Cu+1 Copper(I) -1 Anion Wildcard Fe+2 Iron(II) Li+1 Lithium +2 Cation Wildcard P-3 Phosphide S-2 Sulfide Ca+2 Calcium O-2 Oxide Cu+2 Copper(II) +1 Cation Wildcard Li+1 Lithium Li+1 Lithium F-1 Fluoride Ti+4 Titanium (IV) S-2 Sulfide Na+1 Sodium Al+3 Aluminum(III) -2 Anion Wildcard Na+1 Sodium F-1 Fluoride