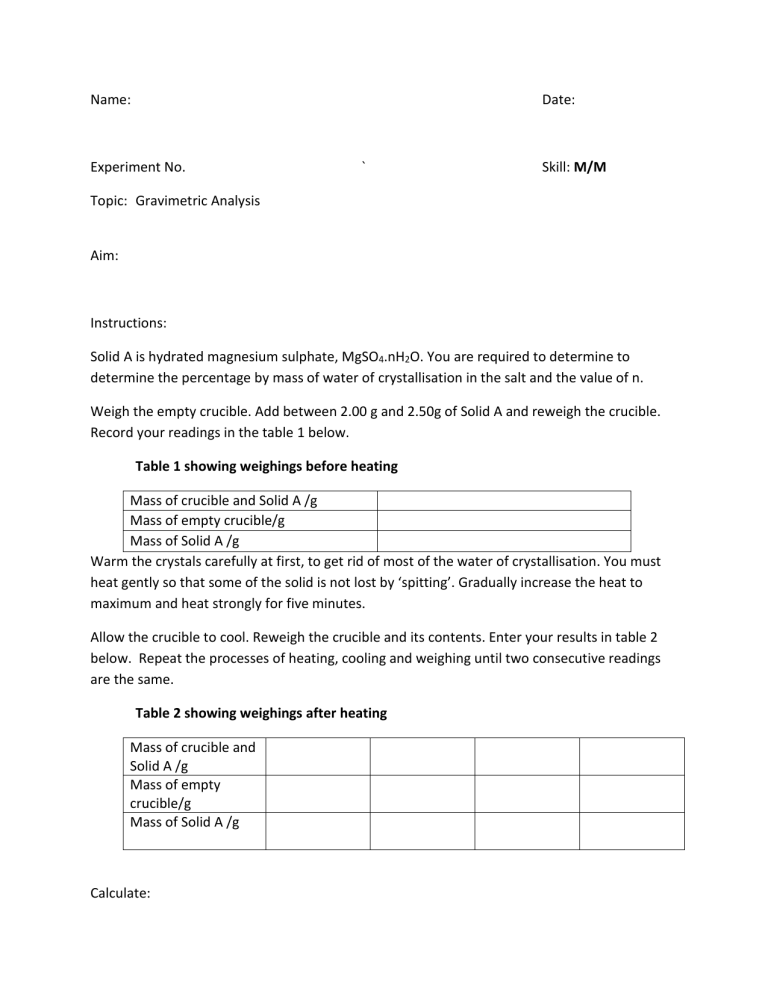
Name: Date: Experiment No. ` Skill: M/M Topic: Gravimetric Analysis Aim: Instructions: Solid A is hydrated magnesium sulphate, MgSO4.nH2O. You are required to determine to determine the percentage by mass of water of crystallisation in the salt and the value of n. Weigh the empty crucible. Add between 2.00 g and 2.50g of Solid A and reweigh the crucible. Record your readings in the table 1 below. Table 1 showing weighings before heating Mass of crucible and Solid A /g Mass of empty crucible/g Mass of Solid A /g Warm the crystals carefully at first, to get rid of most of the water of crystallisation. You must heat gently so that some of the solid is not lost by ‘spitting’. Gradually increase the heat to maximum and heat strongly for five minutes. Allow the crucible to cool. Reweigh the crucible and its contents. Enter your results in table 2 below. Repeat the processes of heating, cooling and weighing until two consecutive readings are the same. Table 2 showing weighings after heating Mass of crucible and Solid A /g Mass of empty crucible/g Mass of Solid A /g Calculate: 1. Mass of water lost due to heating 2. Mass of anhydrous magnesium sulphate 3. Percentage , by mass, of water of crystallisation present in solid A 4. The moles of water lost due to heating 5. The moles of anhydrous magnesium sulphate 6. The value of n Discussion: Explain the principles of Gravimetric analysis with reference to this experiment. .................................................................................................................................................... .................................................................................................................................................... .................................................................................................................................................... .................................................................................................................................................... .................................................................................................................................................... .................................................................................................................................................... .................................................................................................................................................... Sources of Error: .................................................................................................................................................... .................................................................................................................................................... .................................................................................................................................................... .................................................................................................................................................... .................................................................................................................................................... .................................................................................................................................................... .................................................................................................................................................... Precautions: .................................................................................................................................................... .................................................................................................................................................... .................................................................................................................................................... .................................................................................................................................................... .................................................................................................................................................... .................................................................................................................................................... ....................................................................................................................................................




