AP Chemistry Summer Assignment
advertisement

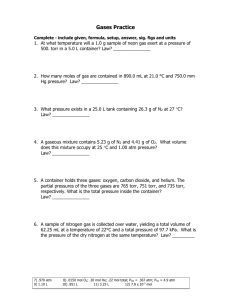
AP Chemistry Summer Assignment Summer 2014 All documents will be linked to the class web-site. As well, the answers will be linked for the practice test, but not the solutions. Solutions by you the students will be required to receive credit for the work. Part 1 Sketch all the lab equipment listed below and identify each piece’s purpose. There are websites that contain this information, you need to search for them. 1. Erlenmeyer Flask 12. Filter Paper 24. Crucible Tongs 2. Pasteur Pipette 13. Evaporating Dish 25. Thermometer 3. Glass Stir Rod 14. Watch Glass 26. Wire Gauze 4. Volumetric Flask 15. Mortar and Pestle 27. Wash Bottle 5. Beakers 16. Crucible and Lid 28. Wire Brush 6. Graduated Cylinder 17. Clay Triangle 29. Beaker Tongs 7. Test Tubes 18. Buret Clamp 30. Spark Lighter 8. Rubber Stoppers (with and without 19. Utility Clamp 31. Metal Scoop holes) 20. Ring Stand 9. Eudiometer 21. Ring Clamp 10. Buret 22. Bunsen Burner 11. Funnel 23. Test Tube Clamp Memorize the names and formulas of the common polyatomic ions, on page 65 of the book (Table 2.5, Common Polyatomic Ions). I recommend making flashcards and/or use Quizlet. Please keep your flashcards, as you will be tested on them throughout the entire year. There will be a test on the FIRST DAY OF SCHOOL on the polyatomic ions! Part 2 Complete the practice test by the first day of class. There will be a test on this material within the first two weeks of school Summer Review Practice Test 1. Answer on a separate sheet of paper. Show all work (for problems that require it) 2. Determine the number of significant figures in the following numbers. A. 0.02 D. 501.0 B. 0.020 E. 5000 C. 501 F. 5000. 3. In your laboratory experiment, you determine that the molar mass of a compound is 34.45 grams/mol. The actual molar mass of this compound is 43.25 grams/mol. What is your percent error? 4. How many protons, neutrons, and electrons do the following atoms or ions have? A. Ca D. Fe3+ B. I E. carbon-13 C. uranium 5. Identify the type of bond in the following compounds (ionic, covalent, metallic): A. CO2 C. Fe-Fe B. MgCl2 D. FeO 6. Name the following ionic compounds: A. NaCl B. MgBr2 A. B. C. Formula Work with units Answer with correct units C. D. FeO Co(NO3)2 7. 8. Name the following covalent compounds: A. CO2 B. C2H4 C. D. N2O3 SCl6 9. Write the formulas for the following ionic compounds (using the swap ‘n drop method): A. calcium chloride C. potassium nitrate B. iron (III) oxide D. titanium (IV) phosphate Write the formulas for the following covalent compounds: A. diphosphorus pentoxide B. tricarbon octahydride C. carbon monoxide 10. How many atoms are in 1.2 mol of water? 11. How much does 6.42 mol of CH4 weigh? 12. How many atoms are in 0.0034 grams of NaCl? 13. Balance the following equations A. AlBr3 + K2SO4 KBr + Al2(SO4)3 B. HCl + CaCO3 CaCl2 + H2O + CO2 C. C4H8 + O2 CO2 + H2O 14. Balance the following equation, and answer the questions: Zn + HCl ZnCl2 + H2 A. How many moles of hydrogen gas can be produced from 2.34 moles of zinc metal? B. How many moles of zinc chloride can be produced from 2.34 grams of zinc metal? C. How many grams of hydrochloric acid do you need to react completely with 2.34 grams of zinc? 15. How many grams of KNO3 should be used to prepare 100.0 mL of a 0.500 M solution? 16. Change the following temperatures from Celcius and Kelvin, and vice versa: A. -34.5°C K B. 12 K °C 17. Covert the following units of pressure: A. 721 torr atm B. 0.034 kPa torr 18. If a sample of gas occupies 3.0 L under 1.5 atm of pressure, at 20.0°C, what volume will it occupy at 2.5 atm and 30°C? 19. How many moles of oxygen will occupy a volume of 2.5 L at 1.2 atm and 25°C? 20. Find the number of grams of CO2 that exert a pressure of 785 torr at a volume of 323 mL and a temperature of 32°C. 21. Write the formulas for the following polyatomic ions. A correct formula must have the correct charge. A. Nitrate J. Permanganate R. Hydrogen phosphate B. Carbonate K. Perchlorate S. Peroxide C. Phosphate L. Chlorite T. Dichromate D. Sulfate M. Bicarbonate (hydrogen U. Acetate E. Sulfite carbonate) V. Chlorate F. Nitrite N. Thiocyanate W. Ammonium G. Chromate O. Mercury (I) X. Hydroxide H. Hypochlorite P. Hydrogen sulfate Y. Dihydrogen phosphate I. Oxalate Q. Cyanide C. D. 1.53 atm mm Hg 342.34 mm Hg torr