GASES WORKSHEET
advertisement
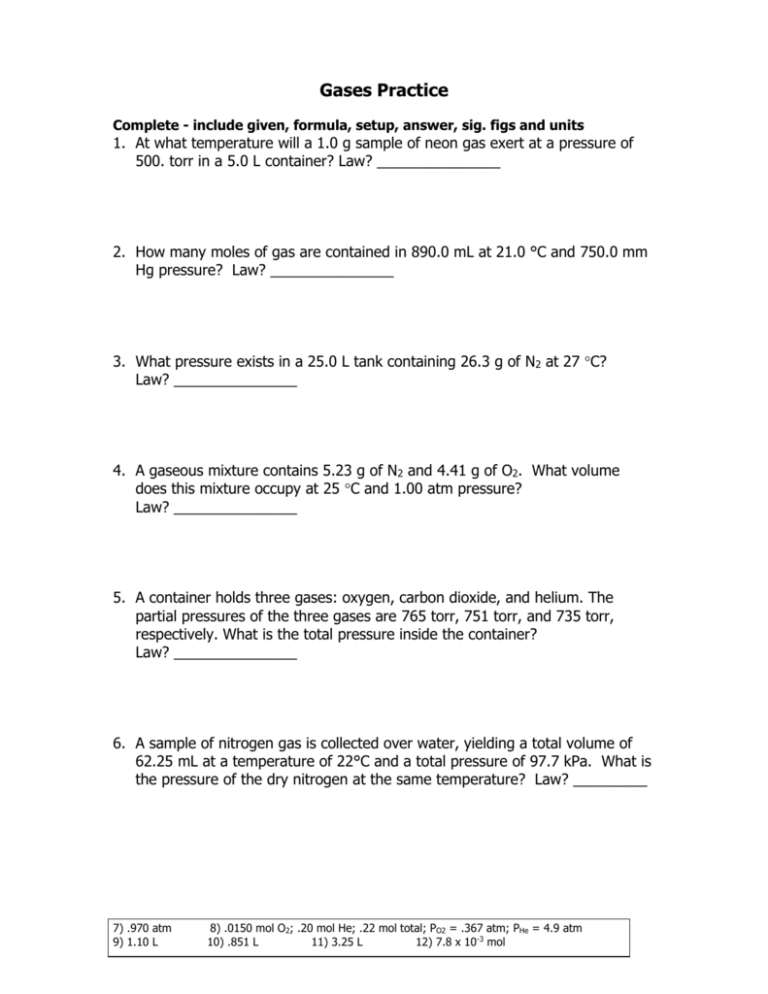
Gases Practice Complete - include given, formula, setup, answer, sig. figs and units 1. At what temperature will a 1.0 g sample of neon gas exert at a pressure of 500. torr in a 5.0 L container? Law? _______________ 2. How many moles of gas are contained in 890.0 mL at 21.0 °C and 750.0 mm Hg pressure? Law? _______________ 3. What pressure exists in a 25.0 L tank containing 26.3 g of N2 at 27 C? Law? _______________ 4. A gaseous mixture contains 5.23 g of N2 and 4.41 g of O2. What volume does this mixture occupy at 25 C and 1.00 atm pressure? Law? _______________ 5. A container holds three gases: oxygen, carbon dioxide, and helium. The partial pressures of the three gases are 765 torr, 751 torr, and 735 torr, respectively. What is the total pressure inside the container? Law? _______________ 6. A sample of nitrogen gas is collected over water, yielding a total volume of 62.25 mL at a temperature of 22°C and a total pressure of 97.7 kPa. What is the pressure of the dry nitrogen at the same temperature? Law? _________ 7) .970 atm 9) 1.10 L 8) .0150 mol O2; .20 mol He; .22 mol total; PO2 = .367 atm; PHe = 4.9 atm 10) .851 L 11) 3.25 L 12) 7.8 x 10-3 mol 7. Hydrogen gas is collected by water displacement. Total volume collected is 0.461 L at a temperature of 17°C and a pressure of 0.989 atm. What is the pressure of dry hydrogen gas collected? Law? _______________ 8. A 1.00 L at 25 °C tank contains .480 grams of oxygen and .8000 grams of helium. Calculate the following. How many moles of O2 are in the tank? How many moles of He are in the tank? Total moles of gas in tank. Partial pressure of O2. Partial pressure of He. 9. If 1.39 g of carbon monoxide is reacted with oxygen, what volume of carbon dioxide is produced at 12.3oC at 107.4KPa? 10. Zinc metal reacts vigorously with hydrochloric acid to form hydrogen gas and zinc chloride. What volume of hydrogen gas at 65 C and 1.01 atm is formed from the reaction of 2.03 g of zinc in excess hydrochloric acid? 11. Ammonium chloride, when heated decomposes into hydrochloric acid and ammonia gas. If 5.03 g of ammonium chloride decomposed completely what volume of ammonia gas would be collected at 24 C and 535 mm Hg. 12. How many moles of C8H18 (octane) are required to fill a 1.4 dm3 airbag with CO2 if the wrecker truck burns octane at STP? (Exhaust fumes are used to fill airbags to upright flipped tractor trailers.) 1) 8.0 x 102 K 5) 2251 torr 2) .03640 mol 6) 95.1 kPa 3) .925 atm 4) 7.95 L











