Chemistry Unit

Chemistry Unit
Atom-
Compound-
Molecule-
Octet Rule-
Noble Gas-
Polymer- (∆-∆-∆-∆)
Monomer-
Covalent bond-
Ionic bond-
Atomic Number-
Valence Shell-
Valence Electron-
Period-
Group/Family-
Isotope-
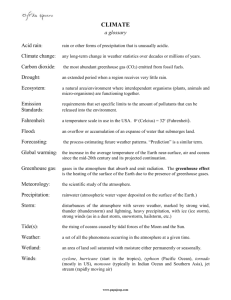
Acids and Bases
0
Acids
Rain with a pH lower than
Acid Rain is made from -
Acid-
Base-
Base
14
Combustion-
Coal –
Chemical Energy –
Polymers
Monomer –
Polymer –
Cross linking –
Synthetic –
Polyethylene –
Linear polymer –
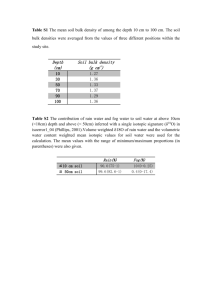
Our Earth’s Atmosphere
Radiation-
Convection-
Conduction-
Electromagnetic Energy -
Ozone- O
3
,
CloroFlouroCarbons (CFC)
Greenhouse Gasses-
CO
2
-
Greenhouse effect
Relative Humidity –
Dew Point -
More Atmosphere
Water Cycle
Radiation -
Evaporation –
Condensation –
Precipitation –
Infiltration –
Run-off –
Groundwater Flow –
Transpiration –
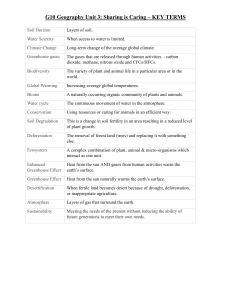
Water Systems Unit
Soil Horizons- The different layers of soil. First is the top that we see with the leaves.
Second is the black dark soil. Third is the mineral soil which is yellow/brown
Watershed/Drainage Basin-
Divide-
Discharge-
Tributary-
Lake-
Pond-
Buffer Zone –
Eutrophication-
Wetland-
Porosity-
Zone of Saturation-
Water Table-
Permeability-
Aquifer -
Drawdown-
Recharge-
Well-
Countour line –
Contour interval –
Nitrate –
Phosphate –
Electricity
Importance of Electrons –
Light Bulb Function –
Resistor –
Conductor –
Insulator –
Series circuit –
(diagram)
Parallel circuit –
(diagram)
Ohm’s Law –
Voltage –
Current –
Resistance -