3.7 Evaporation and distillation
advertisement

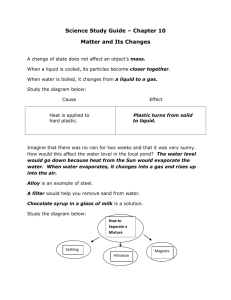
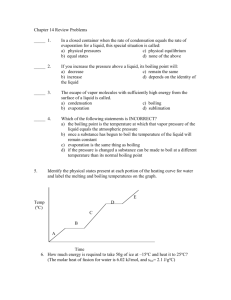
3. 7 EVAPORATION AND DISTILLATION What is the difference between evaporation and boiling? A liquid can evaporate at any temperature, but boiling occurs only at one particular temperature called the boiling point. Evaporation is slow at low temperatures, but fast at the boiling point. What other things affect the rate at which evaporation occurs? Factors that affect the rate of evaporation. When you hang out your clothes to dry, what can you do to help the water evaporate quickly? There are three things you can do: (i) You can hang the clothes in a warm place. As we just saw, temperature is one factor that affects the rate of evaporation. The higher the temperature, the faster the clothes dry. (ii) You can spread the clothes out to expose the biggest possible area to the air. The area exposed is a factor that affects the rate of evaporation. The larger the area exposed, the faster the clothes dry. Three simple experiments For each experiment, you will need two equally wet pieces of paper. In each case (i) find out which one dries first, and (ii) decide which factor made the water evaporate faster. 1. Spread out one piece of paper in a warm place and one in a cool place. Both places must be equally windy. 2. Fold one piece of paper into quarters, but spread out the other one. Place them side-by-side in the same warm place. 3. Spread out one piece of paper in a calm place and one in a windy place. Both places must be equally warm. (iii) You can hang the clothes in a place where the air is moving. The wind is the third factor that affects the rate of evaporation. The stronger the wind, the faster the clothes dry. Distillation. How can we obtain really pure water? If the water looks dirty we can filter it. But even then, there may be something dissolved in the water. Remember, a solute passes through the filter paper with the solvent. A good way to find out if a solute is present is to evaporate the water. You can try this with the water you use at home or at school. Leave a clean plate full of water out in the sun to evaporate. When the water has completely evaporated, look closely at the plate. Has any solute been left on the plate? In fact most of the water we use has some minerals from the earth dissolved in it. One way to obtain really pure water is to distil it. The impure water is boiled in a flask. Only the water evaporates into steam. Any solute or suspension stays in the flask. The steam passes through a tube called a condenser which has cold water flowing over it. As it cools, the steam condenses into pure water. This process of evaporation and condensation is called distillation. Even sea water, which contains a lot of salt, can be fully purified by distillation. Pure water obtained in this way is called distilled water. 1. What are the three factors which speed up evaporation? What does the word factor mean? 3. What is distillation? How does a Liebig condenser work? 2. In each of the experiments described above, why must the two pieces of paper be equally wet? 4. A mixture of two miscible liquids with different boiling points can be separated by distillation. Try to explain how you think this works. Why must the temperature be carefully controlled? 3-7