Separating Solutions - Grade 7 Science is Awesome!
advertisement

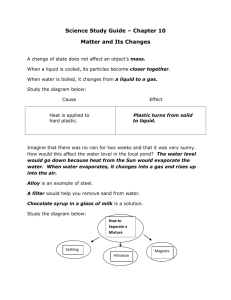
Grade 7 Science Pure Substances and Mixtures Ms. Willis Learning Goals / Success Criteria I can describe the processes used to separate solutions into their components and identify some industry applications of these processes. Vocabulary: evaporate, evaporation, distillation Particles of Solutions Recall that solutions are mixtures in which the particles of the different components are so completely mixed that the mixture appears to be a single substance. E.g.: Salt water in the ocean….. How can we separate Solutions? 1. Evaporation 2. Distillation Evaporation Definition: the process by which a sample of matter changes from a liquid to a gas. The solid (or a more concentrated solution) is left behind. Evaporation is often used to remove the liquid from a solution made of a liquid and a solid. Evaporation Maple syrup is made by boiling the sap which evaporates the water and leaves the concentrated solution (syrup) or sugar behind. Releasing water vapor into the air is not harmful, however, burning fossil fuels to make the heat can be polluting. Distillation Definition: the process of separating liquids in a solution by heating the solution, trapping and cooling the gas, and collecting the resulting pure liquid. Short video to illustrate: https://www.youtube.com/watch?v=xxNfJLMNS4E If you had a liquid solution with more than two liquid components, you could repeat the distillation process to separate all the liquid parts of the solution and use a different collecting flask for each component. Distillation Distillation Picture of a seawater distillation plant: (Seawater must go through several stages to become drinking water.) Distillation New technologies are going beyond simple distillation for “desalinating” water: A closer look at desalinating seawater: https://www.youtube.com/watch?v=aysj7696b0A